|
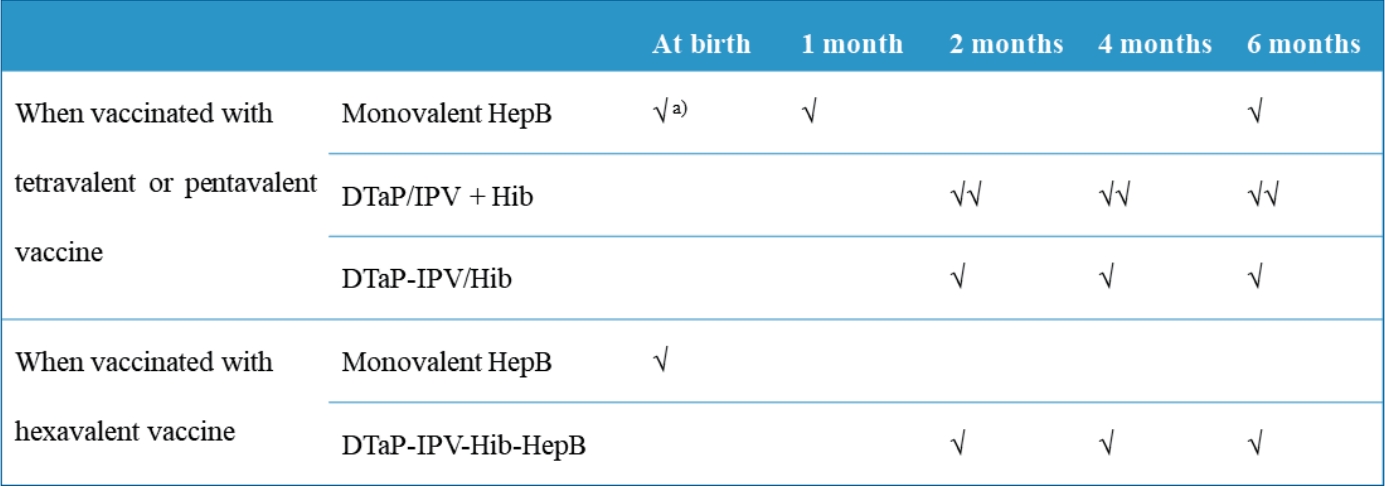
∙ Diphtheria and tetanus toxoids and acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b-hepatitis B (DTaP-IPV-Hib-HepB) was licensed in Korea in April 2020.
∙ DTaP-IPV-Hib-HepB is indicated as a 3-dose primary series for infants aged 2, 4, and 6 months who received the standalone HepB vaccine at birth.
∙ Infants born to HepB surface antigen-positive mothers are currently recommended to be immunized with HepB immunoglobulin at birth and then monovalent HepB vaccine at 0, 1, and 6 months. |






