Article Contents
| Clin Exp Pediatr > Volume 57(7); 2014 |
|
Abstract
Benign convulsion with mild gastroenteritis (CwG) is a type of afebrile seizure that occurs in children. CwG is defined as a convulsion in a previously healthy child with no known central nervous system infection or encephalopathy, accompanying mild diarrhea without fever, electrolyte imbalance, or moderate to severe dehydration. Convulsions in CwG are characterized by multiple brief episodes of generalized or focal seizures. Although the etiology and pathophysiology have yet to be fully explained, many pathogenic mechanisms have been proposed including the possibility of direct invasion of the central nervous system by a gastrointestinal virus such as rotavirus or the possibility of indirect influence by the production and effects of certain mediators. The electroencephalogram findings are benign and long-term antiepileptic treatment is typically not required. Long-term prognosis has been favorable with normal psychomotor development. This review provides a general overview of CwG with the goal of allowing physicians practicing in the field of pediatrics to better recognize this unique entity and, ultimately, to minimize unnecessary evaluation and treatment.
In 1982, Morooka1) first reported a clinical condition in which mild viral gastroenteritis without severe dehydration, electrolyte imbalance, or hypoglycemia could trigger afebrile convulsions. In 1995, Komori et al.2) further characterized "benign convulsion with mild gastroenteritis (CwG)" as (1) previously healthy infants and young children aged 6 months to 3 years having afebrile brief generalized tonic-clonic convulsions between the first and the fifth sick day of viral gastroenteritis usually in winter, (2) mild dehydration (<5%), (3) seizures tending to occur repetitively over several days, (4) an interictal electroencephalogram (EEG) showing no epileptic discharges, (5) frequent detection of rotavirus antigen in the stool, (6) normal other laboratory examinations including cerebrospinal fluid, serum electrolytes, and blood glucose, and (7) always a good prognosis. Although CwG has not been categorized by the International League Against Epilepsy (ILAE)3), this distinct clinical entity is now widely recognized worldwide as situation-related seizures rather than epilepsy. In more recent years, numerous reports and studies have described its characteristics mainly from East Asian countries, such as Japan, Korea, and Taiwan4,5,6,7,8,9,10,11,12), and recently from Western countries13,14,15,16,17,18). CwG is now defined as a convulsion accompanying symptoms of mild diarrhea without moderate to severe dehydration, electrolyte imbalance, and fever before and after the seizures in previously healthy infants and children without meningitis, encephalitis, or encephalopathy19). This review covers an overview on the epidemiology, clinical characteristics, pathophysiology, diagnosis and differential diagnosis, treatment, and prognosis of CwG in order to provide insight into a rather unrecognized entity of seizure commonly encountered in clinical practice.
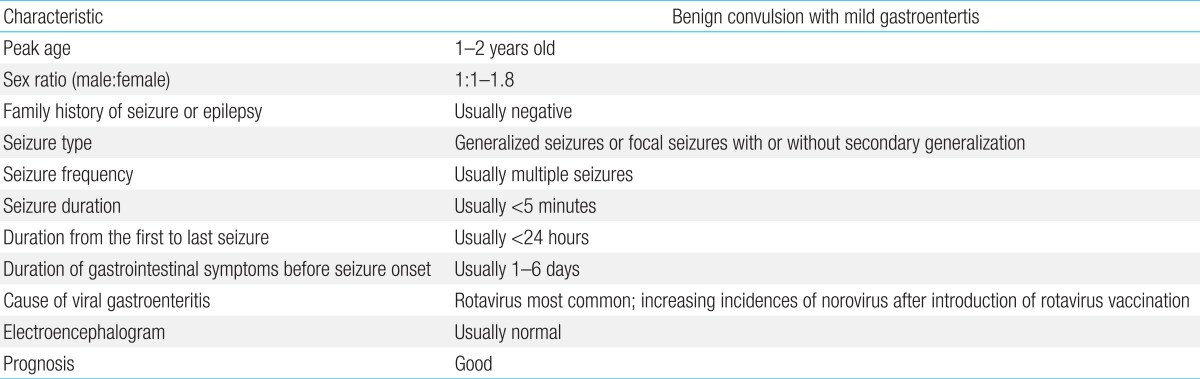
CwG is known to occur predominantly in winter and early spring in temperate countries, corresponding to the outbreaks of viral gastroenteritis-especially rotavirus gastroenteritis4,5,12). The incidence of CwG associated with rotavirus gastroenteritis is approximately 2%-3%5,12,17). CwG occurs in children aged 1 month to 6 years, peaking from 1 to 2 years4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19). Studies have shown that its incidence is similar between both sexes. Meanwhile, there are also reports of female dominance in incidence, with a male to female ratio of 1:1.5-1.85,12). The family history of epilepsy is usually negative in CwG. However, according to a recent study, the family history of epilepsy in the first and second-degree relatives has been reported up to 16.4%18). The predominance of reports and studies from East Asian countries suggests that CwG may be predominant in certain ethnicities13). Recently, reports and studies from Western countries have increased, indicating that CwG is a worldwide condition instead of being confined to East Asia18).
Although the majority of seizure types associated with CwG are known to be generalized seizures, focal features-such as lateral eye deviation and hemiconvulsion or complex partial seizures with loss of responsiveness without convulsive movements-have also been reported2,4,12,14,18). According to previous studies, focal or partial types of seizures were observed in 13%-65% of cases2,4,12,14,18).
Another unique characteristic of CwG is that multiple seizures usually occur during the illness, ranging from 1-8 episodes within a 24-hour period4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19). In some studies, ≥2 seizures developed in 57%-88% of patients with CwG4,5,7,11,12,18). The mean frequency of seizures in CwG was 2.4-3.0 episodes (range, 1-8 episodes)2,7,11,12). One study reported a significantly higher frequency of seizures in patients aged ≥2 years than in those aged <2 years5).
Although some studies have described that seizures lasted >24 hours in 5%-8% of patients, most seizures usually ceased within 24 hours4,12). The mean time from the seizure onset to the last seizure in patients with multiple seizures was 7.7-8.6 hours (range, 0.5-48 hours) according to previous studies4,12).
The duration of the seizures in CwG is known to be brief, with the seizures usually lasting from 30 seconds to <5 minutes4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19). However, some studies have reported seizures lasting >30 minutes10,12,16,19). The duration between the onset of gastrointestinal symptoms and seizures usually ranges from 1-6 days (mean, 2.3-3.8 days)4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19). Meanwhile, some studies have reported that the seizures occurred before the onset of diarrhea2,16,18).
The characteristics of CwG are summarized in Table 1.
Although the mechanism associated with the seizures in CwG patients remains unknown, an interesting finding of CwG is that it only occurs in infants and young children. Moreover, the rapid development and growth of the central nervous system (CNS) in this age group, which is similar to febrile seizures where brain immaturity plays a role, may increase the susceptibility to the development of seizures in CwG19).
Among the many organisms causing gastroenteritis, rotavirus has been frequently detected in the stool specimens of CwG patients2,4,8,13,14,18). Many studies have investigated the relationship between a rotavirus infection and its effect on the CNS. One hypothesis is that rotavirus may directly invade the CNS via the bloodstream and consequently cause encephalopathy, encephalitis, or seizures20,21). Systemic infection by rotavirus has been confirmed by the detection of rotavirus antigens and RNA in serum specimens of children with rotavirus gastroenteritis22,23,24). Moreover, some studies have detected rotavirus RNA in the cerebrospinal fluid of patients with gastroenteritis and seizures25,26). In vitro, neuronal infection and replication by rotavirus has been demonstrated, with the identification of NSP4 in dendritic processes27). NSP4 is a glycosylated protein of rotavirus, which is known to play a major role in the pathogenesis of rotavirus in gastrointestinal epithelial cells via alteration of calcium homeostasis in infected cells28). Moreover, NSP4 is known to possess membrane-destabilizing properties29). Therefore, the direct invasion of rotavirus into the CNS may induce seizures in CwG by replicating and inducing the production of NSP4 resulting in neurotoxicity and neurotransmitter dysregulation. However, this hypothesis is controversial as rotavirus RNA has not been detected in all cases.
Another hypothesis is that rotavirus may induce seizures indirectly by increased circulation of various mediators produced in the gastrointestinal tract. During inoculation and replication of rotavirus in the intestine, intracellular calcium influx induced by NSP4 may induce cell death of enterocytes and stimulate the release of cytokines, prostaglandins, reactive oxygen species, and even NSP4 itself30). Serum calcium levels have been found to be significantly lower in rotavirus gastroenteritis patients with seizures than in patients without seizures, indicating that the disruption of calcium homeostasis plays a significant role in the generation of seizures in rotavirus gastroenteritis31). Moreover, cytokines may play a role in triggering the seizures. Several studies have suggested that cytokines such as interleukin (IL) 8 and IL-10 may be involved in the pathogenesis of rotavirus infection, and that increased systemic spread of the virus may stimulate a strong immune response in the host24,32,33,34). Furthermore, nitric oxide may play a role in the indirect mechanism. According to a study, nitric oxide was significantly elevated in the cerebrospinal fluid of patients with rotavirus-induced seizures compared to the level in patients with purulent meningitis, encephalitis, or febrile convulsions35). Therefore, increased circulation of mediators such as NSP4, cytokines, and nitric oxide during systemic rotavirus infections may result in them to cross the blood-brain barrier and induce seizures in CwG patients.
Particular rotavirus genotypes may be associated with the seizures observed in CwG patients. Two studies have been reported in Korea with controversial results36,37). Choi et al.36) reported that there was a statistical significance in the detection rate of the genotype P[4]G2 in stool specimens between seizure-free patients and CwG patients with rotavirus gastroenteritis. Meanwhile, Yang et al.37) reported that P[8]G3 was dominant in patients with CwG. However, there was no statistical significance in the detection rate of particular genotypes between groups. Therefore, the occurrence of rotavirus-associated CwG may not be genotype-specific. Further large-scaled studies are required in the future to clarify this association.
Despite many studies investigating the association between rotavirus and CwG, gastroenteritis in CwG is not solely confined to rotavirus infections. The detection of other gastrointestinal viruses such as norovirus, sapovirus, adenovirus, and coxsackievirus in stool specimens of patients with CwG has been reported8,10,38). According to Chen et al.10), the incidence of convulsions associated with norovirus infections was higher than that associated with rotavirus infections (29.7% vs. 5%), highlighting norovirus as a significant pathogen in CwG-in the postrotavirus vaccination era. Further studies investigating the association between CwG and pathogens other than rotavirus, especially norovirus, are required in the future.
Some have postulated that CwG should be classified into the spectrum of benign infantile seizures because of the overlapping clinical picture39,40,41). Moreover, genetic studies have supported this postulation, proposing a channelopathy of genetic heterogeneity in benign infantile seizures as a possible cause of CwG41,42,43,44). Furthermore, the similarity of benign infantile seizures and CwG with regard to the effectiveness of low-dose carbamazepine therapy have indicated the possibility of sodium channel abnormalities such as SCN1A mutations in CwG9,45). The identification of de novo mutations of the SCN1A gene in 11 of 14 patients with alleged vaccine encephalopathy have supported this hypothesis46). However, a recent study in Taiwan reported no SCN1A mutation in 12 patients with CwG47). Further investigation is required in order to verify whether CwG is associated with sodium channelopathies and whether it is a marginal syndrome of benign infantile seizures.
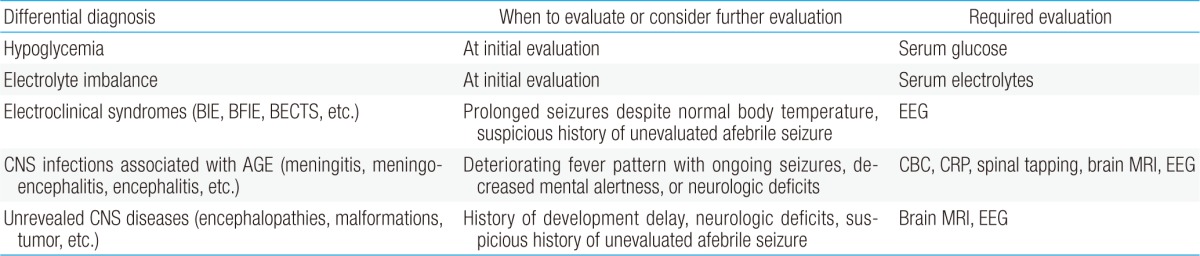
The diagnosis of CwG is made on clinical grounds. All infants and children are previously healthy with normal psychomotor development and without any previous history of CNS diseases or disorders. Dehydration due to diarrhea is only mild and laboratory tests show no derangement in electrolyte levels. Therefore, initial laboratory tests, including serum glucose, blood urea nitrogen, creatinine, and electrolytes, are required in order to assess the degree of dehydration and to differentiate CwG from seizures due to hypoglycemia or electrolyte imbalances.
Although fever is common during viral gastroenteritis and therefore can occur during CwG, the body temperature is <38℃ during seizure episodes in CwG. A history of prolonged fever with deteriorating neurologic signs and symptoms should raise the possibility of a more severe and progressive CNS infection associated with viral gastroenteritis. Neurological manifestations other than CwG such as meningoencephalitis, encephalopathy, acute necrotizing encephalitis, meningitis, Reye syndrome, and flaccid paralysis have been associated with 2%-5% of rotavirus infections, with a presentation that may mimic CwG in some cases48,49). Complete blood cell counts, serum C-reactive protein, spinal tapping, magnetic resonance imaging, and EEG should be strongly considered at initial presentation and at any time during the course in patients with deteriorating fever patterns, and ongoing seizures, decreased mental alertness, or neurologic deficits.
Although interictal EEG findings are usually normal in CwG4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19), some patients may initially present with abnormal EEG findings, such as slow waves, focal spikes, or epileptiform discharges5,6,7,10,11,16,18). Despite some abnormalities, most EEG findings return to normal during the follow-up period. Therefore, the role of an EEG is of limited value in the evaluation of CwG. However, the first onset of electroclinical syndromes such as benign infantile epilepsy, benign familial infantile epilepsy, and benign epilepsy with centrotemporal spikes may also coincidentally occur with the onset of viral gastroenteritis, mimicking CwG. Therefore, an EEG should be obtained in patients with prolonged seizures despite normal body temperature during the course of the disease or a suspicious history of unevaluated afebrile seizure. The evaluation required for the differential diagnosis of CwG is summarized in Table 2.
Long-term antiepileptic treatment is not required for patients with CwG. The seizures in CwG do not usually persist after the illness and the role of antiepileptic treatment may be limited to stopping them during the illness. Some studies have suggested that most seizures in CwG would end within 24 hours, and intensive antiepileptic treatment such as high dose phenobarbital, continuous infusion of midazolam, and continuous pentobarbital would not be recommended in patients with CwG4). Several studies have reported that treatment with carbamazepine or lidocaine may be effective in the cessation of the seizures associated with CwG, whereas benzodiazepines are ineffective9,45,50). Recently, a low dose of carbamazepine of 5 mg/kg once per day has been shown to be effective in treating rotavirus-associated CwG9). Further large-scaled prospective randomized studies are required to determine the optimal treatment for CwG.
Despite repetitive seizures that may last for several days, the prognosis of CwG is generally favorable. Patients do not develop epilepsy in the future, and psychomotor development is normal. Therefore, long-term antiepileptic treatment is not required in children with CwG.
According to a recent study reported in the United States, a full course of rotavirus vaccination was associated with statistically significant reductions in the risk of childhood seizures during the year following the last rotavirus vaccination51). Therefore, rotavirus vaccination may reduce the occurrence of CwG by preventing rotavirus infections. Further worldwide studies are required in the future.
CwG is a unique type of afebrile seizure that is associated with mild viral gastroenteritis in previously healthy infants and children without CNS infections and encephalopathy. It is characterized by afebrile brief generalized or focal convulsions. Rotavirus is widely known as the most common virus associated with the occurrence of CwG. Although its pathophysiology is not yet fully understood, direct or indirect effects of gastrointestinal viruses on the CNS have been proposed as possible pathogenic mechanisms. Most EEG findings are normal and long-term antiepileptic treatment is usually not required. Prognosis is always favorable, with normal psychomotor development. Further studies regarding its pathogenesis and other associated viruses are required in the future.
References
1. Morooka K. Convulsions and mild diarrhea. Shonika (Tokyo) 1982;23:131–137.
2. Komori H, Wada M, Eto M, Oki H, Aida K, Fujimoto T. Benign convulsions with mild gastroenteritis: a report of 10 recent cases detailing clinical varieties. Brain Dev 1995;17:334–337.


3. Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 2010;51:676–685.


4. Uemura N, Okumura A, Negoro T, Watanabe K. Clinical features of benign convulsions with mild gastroenteritis. Brain Dev 2002;24:745–749.


5. Hung JJ, Wen HY, Yen MH, Chen HW, Yan DC, Lin KL, et al. Rotavirus gastroenteritis associated with afebrile convulsion in children: clinical analysis of 40 cases. Chang Gung Med J 2003;26:654–659.

6. Cho JH, Joo KE, Kim SK, Shin SH, Lee KH, Yoon HS. Clinical study of benign convulsion with acute gastroenteritis. Korean J Pediatr 2004;47:855–860.
7. Lee JS, Kwon HO, Jee YM, Chae KY. Clinical features of benign infantile convulsions with gastroenteritis. Korean J Pediatr 2005;48:753–759.
8. Kawano G, Oshige K, Syutou S, Koteda Y, Yokoyama T, Kim BG, et al. Benign infantile convulsions associated with mild gastroenteritis: a retrospective study of 39 cases including virological tests and efficacy of anticonvulsants. Brain Dev 2007;29:617–622.


9. Motoyama M, Ichiyama T, Matsushige T, Kajimoto M, Shiraishi M, Furukawa S. Clinical characteristics of benign convulsions with rotavirus gastroenteritis. J Child Neurol 2009;24:557–561.


10. Chen SY, Tsai CN, Lai MW, Chen CY, Lin KL, Lin TY, et al. Norovirus infection as a cause of diarrhea-associated benign infantile seizures. Clin Infect Dis 2009;48:849–855.


11. Lee EH, Chung S. A comparative study of febrile and afebrile seizures associated with mild gastroenteritis. Brain Dev 2013;35:636–640.


12. Kang B, Kim DH, Hong YJ, Son BK, Kim DW, Kwon YS. Comparison between febrile and afebrile seizures associated with mild rotavirus gastroenteritis. Seizure 2013;22:560–564.


13. Narchi H. Benign afebrile cluster convulsions with gastroenteritis: an observational study. BMC Pediatr 2004;4:2



14. Caraballo RH, Ganez L, Santos Cde L, Espeche A, Cersosimo R, Fejerman N. Benign infantile seizures with mild gastroenteritis: study of 22 patients. Seizure 2009;18:686–689.


15. Cusmai R, Jocic-Jakubi B, Cantonetti L, Japaridze N, Vigevano F. Convulsions associated with gastroenteritis in the spectrum of benign focal epilepsies in infancy: 30 cases including four cases with ictal EEG recording. Epileptic Disord 2010;12:255–261.



16. Durá-Travé T, Yoldi-Petri ME, Gallinas-Victoriano F, Molins-Castiella T. Infantile convulsions with mild gastroenteritis: a retrospective study of 25 patients. Eur J Neurol 2011;18:273–278.


17. Lloyd MB, Lloyd JC, Gesteland PH, Bale JF Jr. Rotavirus gastroenteritis and seizures in young children. Pediatr Neurol 2010;42:404–408.


18. Verrotti A, Nanni G, Agostinelli S, Parisi P, Capovilla G, Beccaria F, et al. Benign convulsions associated with mild gastroenteritis: a multicenter clinical study. Epilepsy Res 2011;93:107–114.


19. Verrotti A, Tocco AM, Coppola GG, Altobelli E, Chiarelli F. Afebrile benign convulsions with mild gastroenteritis: a new entity? Acta Neurol Scand 2009;120:73–79.


20. Goldwater PN, Rowland K, Thesinger M, Abbott K, Grieve A, Palombo EA, et al. Rotavirus encephalopathy: pathogenesis reviewed. J Paediatr Child Health 2001;37:206–209.


21. Keidan I, Shif I, Keren G, Passwell JH. Rotavirus encephalopathy: evidence of central nervous system involvement during rotavirus infection. Pediatr Infect Dis J 1992;11:773–775.


22. Blutt SE, Kirkwood CD, Parreno V, Warfield KL, Ciarlet M, Estes MK, et al. Rotavirus antigenaemia and viraemia: a common event? Lancet 2003;362:1445–1449.


23. Fischer TK, Ashley D, Kerin T, Reynolds-Hedmann E, Gentsch J, Widdowson MA, et al. Rotavirus antigenemia in patients with acute gastroenteritis. J Infect Dis 2005;192:913–919.


24. Sugata K, Taniguchi K, Yui A, Miyake F, Suga S, Asano Y, et al. Analysis of rotavirus antigenemia and extraintestinal manifestations in children with rotavirus gastroenteritis. Pediatrics 2008;122:392–397.


25. Nishimura S, Ushijima H, Nishimura S, Shiraishi H, Kanazawa C, Abe T, et al. Detection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcription polymerase chain reaction. Brain Dev 1993;15:457–459.


26. Liu B, Fujita Y, Arakawa C, Kohira R, Fuchigami T, Mugishima H, et al. Detection of rotavirus RNA and antigens in serum and cerebrospinal fluid samples from diarrheic children with seizures. Jpn J Infect Dis 2009;62:279–283.


27. Weclewicz K, Kristensson K, Greenberg HB, Svensson L. The endoplasmic reticulum-associated VP7 of rotavirus is targeted to axons and dendrites in polarized neurons. J Neurocytol 1993;22:616–626.


28. Díaz Y, Chemello ME, Pena F, Aristimuno OC, Zambrano JL, Rojas H, et al. Expression of nonstructural rotavirus protein NSP4 mimics Ca2+ homeostasis changes induced by rotavirus infection in cultured cells. J Virol 2008;82:11331–11343.



29. Tian P, Ball JM, Zeng CQ, Estes MK. The rotavirus nonstructural glycoprotein NSP4 possesses membrane destabilization activity. J Virol 1996;70:6973–6981.



30. Ruiz MC, Cohen J, Michelangeli F. Role of Ca2+in the replication and pathogenesis of rotavirus and other viral infections. Cell Calcium 2000;28:137–149.


31. Yeom JS, Kim YS, Park JS, Seo JH, Park ES, Lim JY, et al. Role of Ca2+ homeostasis disruption in rotavirus-associated seizures. J Child Neurol 2014;29:331–335.


32. Azim T, Ahmad SM, Sefat-E-Khuda , Sarker MS, Unicomb LE, De S, et al. Immune response of children who develop persistent diarrhea following rotavirus infection. Clin Diagn Lab Immunol 1999;6:690–695.



33. Casola A, Garofalo RP, Crawford SE, Estes MK, Mercurio F, Crowe SE, et al. Interleukin-8 gene regulation in intestinal epithelial cells infected with rotavirus: role of viral-induced IkappaB kinase activation. Virology 2002;298:8–19.


34. Jiang B, Snipes-Magaldi L, Dennehy P, Keyserling H, Holman RC, Bresee J, et al. Cytokines as mediators for or effectors against rotavirus disease in children. Clin Diagn Lab Immunol 2003;10:995–1001.



35. Kawashima H, Inage Y, Ogihara M, Kashiwagi Y, Takekuma K, Hoshika A, et al. Serum and cerebrospinal fluid nitrite/nitrate levels in patients with rotavirus gastroenteritis induced convulsion. Life Sci 2004;74:1397–1405.


36. Choi JH, Kim YJ, Oh JW, Kim CL, Yum MK, Sul IJ, et al. Genotype of rotavirus isolated from patients with rotaviral enteritis and neurological complications. Korean J Pediatr 2006;49:513–518.

37. Yang HR, Jee YM, Ko JS, Seo JK. Detection and genotyping of viruses detected in children with benign afebrile seizures associated with acute gastroenteritis. Korean J Pediatr Gastroenterol Nutr 2009;12:183–193.

38. Abe T, Kobayashi M, Araki K, Kodama H, Fujita Y, Shinozaki T, et al. Infantile convulsions with mild gastroenteritis. Brain Dev 2000;22:301–306.


39. Sakai Y, Kira R, Torisu H, Yasumoto S, Saito M, Kusuhara K, et al. Benign convulsion with mild gastroenteritis and benign familial infantile seizure. Epilepsy Res 2006;68:269–271.


40. Okumura A, Watanabe K, Negoro T. Benign partial epilepsy in infancy long-term outcome and marginal syndromes. Epilepsy Res 2006;70(Suppl 1): S168–S173.


41. Specchio N, Vigevano F. The spectrum of benign infantile seizures. Epilepsy Res 2006;70(Suppl 1): S156–S167.


42. Guipponi M, Rivier F, Vigevano F, Beck C, Crespel A, Echenne B, et al. Linkage mapping of benign familial infantile convulsions (BFIC) to chromosome 19q. Hum Mol Genet 1997;6:473–477.


43. Caraballo R, Pavek S, Lemainque A, Gastaldi M, Echenne B, Motte J, et al. Linkage of benign familial infantile convulsions to chromosome 16p12-q12 suggests allelism to the infantile convulsions and choreoathetosis syndrome. Am J Hum Genet 2001;68:788–794.



44. Malacarne M, Gennaro E, Madia F, Pozzi S, Vacca D, Barone B, et al. Benign familial infantile convulsions: mapping of a novel locus on chromosome 2q24 and evidence for genetic heterogeneity. Am J Hum Genet 2001;68:1521–1526.



45. Matsufuji H, Ichiyama T, Isumi H, Furukawa S. Low-dose carbamazepine therapy for benign infantile convulsions. Brain Dev 2005;27:554–557.


46. Berkovic SF, Harkin L, McMahon JM, Pelekanos JT, Zuberi SM, Wirrell EC, et al. De-novo mutations of the sodium channel gene SCN1A in alleged vaccine encephalopathy: a retrospective study. Lancet Neurol 2006;5:488–492.


47. Weng WC, Hirose S, Lee WT. Benign convulsions with mild gastroenteritis: is it associated with sodium channel gene SCN1A mutation? J Child Neurol 2010;25:1521–1524.


48. Ben-Ami T, Sinai L, Granot E. Afebrile seizures and rotavirus gastroenteritis: an infrequently recognized association. Clin Pediatr (Phila) 2007;46:178–180.


49. Hongou K, Konishi T, Yagi S, Araki K, Miyawaki T. Rotavirus encephalitis mimicking afebrile benign convulsions in infants. Pediatr Neurol 1998;18:354–357.





 PDF Links
PDF Links PubReader
PubReader PubMed
PubMed Download Citation
Download Citation


