After gram-positive and -negative bacteria, Candida sepsis is the third most common hospital-acquired infection in neonatal intensive care units (NICUs), accounting for about 12% of cases of late-onset sepsis in very low birth weight infants (VLBWI), weighing less than 1,500 g [1]. The incidence of invasive candidiasis in extremely preterm infants varies among NICUs, reportedly affecting 3%–23% of extremely low birth weight infants (ELBWI), weighing less than 1,000 g at birth and 0%–6% of VLBWI [2].
In extremely preterm infants, invasive procedures, such as endotracheal intubation and central venous catheterization, broad-spectrum antibiotics, parenteral nutrition, and Candida colonization, including the gastrointestinal system, skin, and respiratory tract, are common risk factors for invasive infections [3]. Delayed treatment for candidemia may cause disseminated infection of the end organs, including meningoencephalitis, peritonitis, renal mycetoma, and endophthalmitis [3]. Extremely preterm infants with invasive candidiasis have a high mortality rate of about 30% despite various intensive treatments, including antifungal agents. Those that survive have a higher incidence of neurodevelopmental disorders in infancy than babies without infection [3]. Additionally, infection can lead to longer hospital stays and higher medical expenses [3].
In a recent issue of Clinical and Experimental Pediatrics, Anaraki et al. [4] published a systematic review and meta-analysis on fluconazole prophylaxis (FP) for preventing invasive candidiasis in preterm VLBWI and ELBWI. This article reviewed 16 clinical studies on FP in extremely preterm infants over the last 16 years and compared and summarized the birth weights of the target group, the dosage and dosing interval of the antifungal agent, and the duration of administration. Despite the heterogeneity of comparative variables among clinical trials, extremely preterm infants treated with FP showed lower rates of Candida colonization, invasive infection, and mortality than the control group.
Prior to the introduction of FP, common infection control measures should be established to reduce invasive Candida infection rates among NICU patients. Stewardship on medications, including broad-spectrum antibiotics, antacids, and steroids, and the development of feeding protocols may reduce the risk of Candida infection in preterm infants [3]. Additionally, standardized bundles for inserting and maintaining central lines help reduce central line–associated bloodstream infections, including Candida sepsis [3].
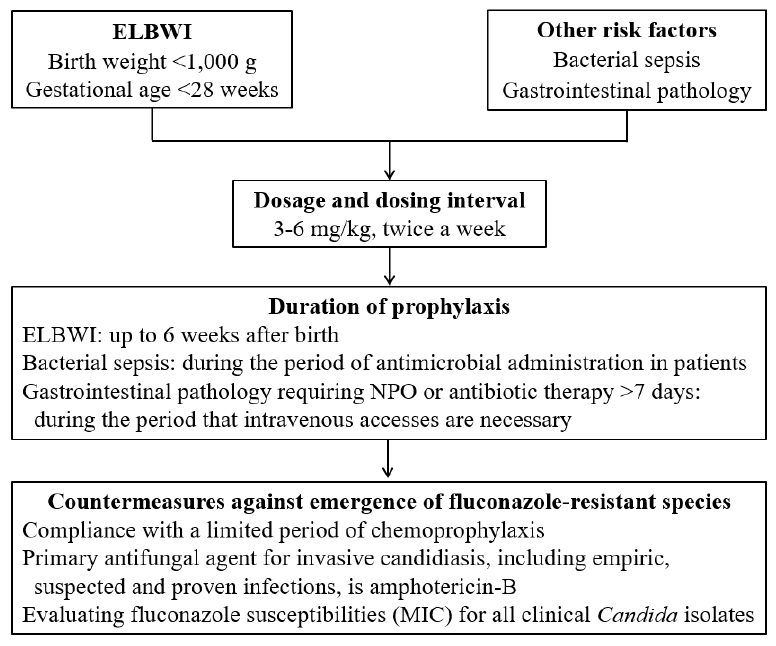
FP is primarily recommended for preterm infants with a birth weight of less than 1,000 g, or a gestational age of less than 28 weeks, in a NICU with a high incidence of invasive candidiasis, affecting more than 5%–10% of all hospitalized patients [3]. Additionally, patients with bacterial bloodstream infections and gastrointestinal pathologies, including congenital anomalies (e.g., tracheoesophageal fistula, gastroschisis, omphalocele, and intestinal atresia), necrotizing enterocolitis, and spontaneous intestinal perforation, may be included in the target group for chemoprophylaxis [5]. Fluconazole doses used for prophylaxis vary from 3–4 mg/kg and up to 6 mg/kg, and the recommended doses have been applied differently among NICUs. According to recent meta-analysis data, when treating extremely preterm infants with FP, both 3 and 6 mg/kg doses were effective at reducing invasive candidiasis and infection-related mortality compared to controls, although an increased dose was not associated with higher efficacy [6]. Therefore, considering the greater potential toxicity and higher cost of increased FP doses, it is better to use the lowest dose (3 mg/kg). Preterm infants (birth weight <1,000 g or gestational age <28 weeks) should receive the prophylaxis from birth until the 6th week of life, but treatment should be discontinued sooner if a baby achieves full enteral nutrition and intravenous access is otherwise unnecessary [3]. Infants with bacterial sepsis may be considered for prophylaxis during treatment with broad-spectrum antibiotics, including third- and fourth-generation cephalosporins, as well as carbapenems [3,5]. Infants with gastrointestinal diseases requiring long-term fasting or antibiotic treatment may be candidates for prophylaxis during the period when intravenous access is necessary [3,5]. When chemoprophylaxis is used in the NICU, countermeasures against the emergence of fluconazole-resistant species should be implemented (Fig. 1) [3,7].
The safety of FP has been evaluated. Cholestasis, which often occurs in extremely preterm infants treated with FP, is associated with underlying diseases during hospitalization, such as total parenteral nutrition, bacterial infections, or necrotizing enterocolitis, and is not related to fluconazole dose or duration [3]. Neurodevelopmental safety has been evaluated in ELBWI receiving chemoprophylaxis. Neurological evaluations performed at 18–22 months and about 8–10 years of age revealed no difference in the rate of neurodevelopmental impairment between the fluconazole and placebo groups [8,9]. Long-term follow-up data of more than 10 years indicated no emergence of native fluconazole-resistant species, including Candida glabrata and Candida krusei, after the introduction of FP in the NICU [7,10].
In conclusion, FP effectively decreases Candida colonization, invasive infection, and mortality in extremely preterm infants. A schedule of lower doses and less frequent administrations of fluconazole for NICU patients is recommended. After the introduction of FP, monitoring the emergence of resistant Candida species and the susceptibility of fluconazole should continue.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


