Article Contents
| Clin Exp Pediatr > Volume 66(1); 2023 |
|
Abstract
Intussusception involves an invagination of the proximal bowel into the distal bowel, with ileocolic intussusception being the most common type. However, a diagnostic delay can lead to intestinal ischemia, bowel infarction, or even death; therefore, its early diagnosis and management are important. The primary role of abdominal radiography is to detect pneumoperitoneum or high-grade bowel obstruction in cases of suspected intussusception, and ultrasonography is the modality of choice for its diagnosis. Nonoperative enema reduction, the treatment of choice for childhood intussusception in cases without signs of perforation or peritonitis, can be safely performed with a success rate of 82%. Enema reduction can be performed in various ways according to image guidance method (fluoroscopy or ultrasonography) and reduction medium (liquid or air). Successful enema reduction is less likely to be achieved in children with a longer symptom duration, younger age, lethargy, fever, bloody diarrhea, unfavorable radiologic findings (small bowel obstruction, trapped fluid, ascites, absence of flow in the intussusception, intussusception in the left-sided colon), and pathological lead points. This review highlights the current concepts of intussusception diagnosis, nonsurgical enema reduction, success rates, predictors of failed enema reduction, complications, and recurrence to guide general pediatricians in the management of childhood intussusception.
Graphical abstract.
Intussusception involves invagination of the proximal bowel into the distal bowel. Ileocolic intussusception is the most common type, comprising more than 80% of cases, while other types include ileoileocolic, enteroenteric, and rarely colocolic intussusceptions [1]. Intussusception typically occurs in infants and toddlers aged 3ŌĆō36 months old [2-5]. The classic clinical presentation is abdominal pain, vomiting, bloody stools, and abdominal mass [6], but it occurs in only 20% of cases, and most children do not show the complete symptom triad (abdominal pain, vomiting, and bloody stool) [7]. The clinical presentation is also age-related; younger children are more likely to experience vomiting, irritability, lethargy, or bloody stools, while older children are more likely to present with abdominal pain [4]. All of these features should warrant a high level of clinical suspicion for intussusception to prevent missing the diagnosis in children with nonspecific symptoms.
According to a nationwide epidemiological study in 2008ŌĆō2016, the incidence of intussusception in children up to 2 years of age was reportedly 196.7 cases per 100,000 in Korea [3]. The overall incidence of intussusception was 28.3 cases per 100,000 person-years in Korea in 2007ŌĆō2017 (Table 1) [5]. Intussusception in preterm infants is extremely rare [8]. According to a systematic review of 24 premature neonates, intussusception is easily confused with necrotizing enterocolitis, the most prevalent diagnosis in preterm infants with abdominal symptomatology; therefore, the accurate diagnosis of intussusception in preterm infants tends to be delayed. In preterm infants, the intussusception usually is located in the small bowel (91%), unlike in infants and children, and a pathological lead point is infrequent. Intussusception is idiopathic in most cases without an identifiable lead point, except the hyperplastic lymphoid tissue in the terminal ileum [1]. Previous studies reported seasonal variations [3,9,10] or no clear seasonal pattern [11,12]. Viral or bacterial gastroenteritis was postulated as a causative factor of intussusception [10,13-15]. Enteric and nonenteric types of adenovirus infection had significant associations in multiple studies, being reported in approximately 30%ŌĆō40% of cases [16-18].
Yoo et al. [19] recently investigated the incidence of monthly visits for intussusception from 7 hospitals in Korea in 2017ŌĆō2020. Interestingly, the incidence of monthly visits has substantially reduced (9.0 to 3.5) after the active implementation of the coronavirus 2019 infection control guidelines initiated in Korea. The review of open data from the Korea Disease Control and Prevention Agency revealed that the incidence of infectious diseases in children significantly decreased in the same period, supporting the hypothesis that viral infection is a cause of intussusception.
The prevalence of pathological lead points has is reportedly 0.3%ŌĆō20% [20]. In a meta-analysis, the pooled proportion of pathologic lead points was 4% in children with intussusception, and MeckelŌĆÖs diverticulum was the most common cause [21]. Other pathological lead points included duplication cyst, polyp, lymphoma, and lymphoid hyperplasia [21]. The incidence of pathologic lead points increases in children older than the typical age groups (>5 years) [20,22]. Children with multiple episodes of recurrent intussusception are at an increased risk of pathologic lead points [22,23] despite most with recurrences not having a pathologic lead point [23].
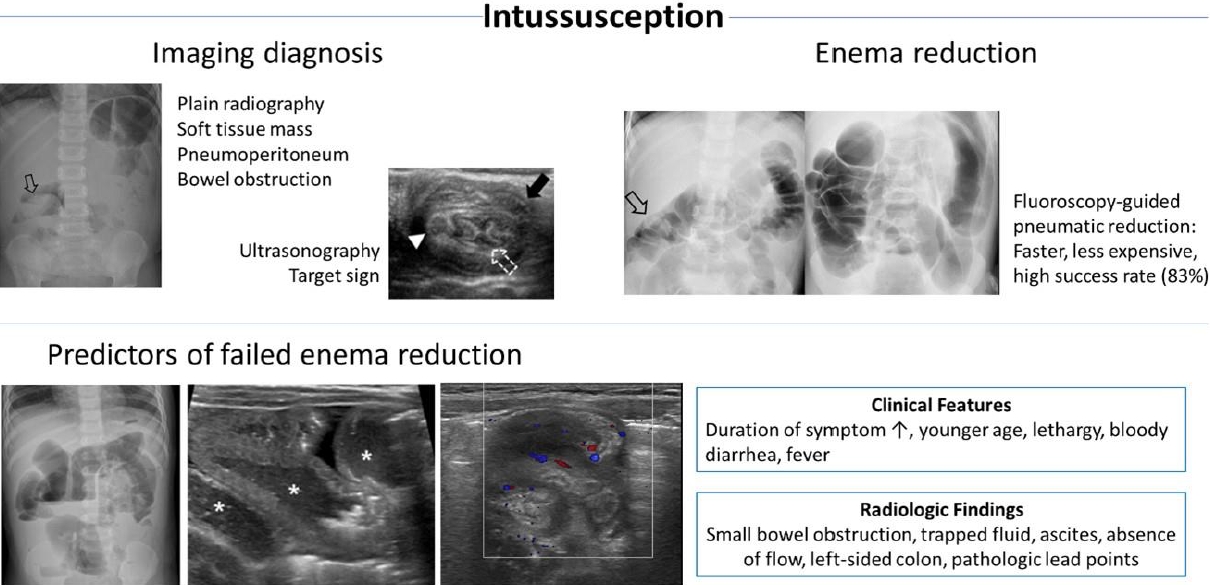
The role of imaging in the diagnosis of intussusception is well established. In cases of suspected intussusception, abdominal radiographs should be evaluated through abdominal supine, erect (preferred when the childrenŌĆÖs condition allows), or left decubitus (for young children unable to be placed erect) views [24,25]. Soft tissue masses along the course of the colon and the absence of large bowel gas in the right iliac fossa are the most specific signs of intussusception (Fig. 1) [26]. Interestingly, deep learning-based algorithms applied in recent studies were demonstrated as feasible for screening pediatric ileocolic intussusception using abdominal radiographs (Table 2) [27,28]. Nevertheless, given the low diagnostic accuracy (40% including equivocal cases [26]) for intussusception, the primary role of abdominal radiographs is to detect pneumoperitoneum or high-grade bowel obstruction [1,29,30]. Pneumoperitoneum on radiographs, peritonitis, and shock are contraindications for nonoperative enema reduction. Children with contraindications to enema reduction should be treated surgically [24,29]. The presence of small bowel obstruction on abdominal radiographs was associated with failed enema reduction in previous studies [31,32], although it is not a contraindication for nonoperative enema reduction.
Ultrasonography is the modality of choice for diagnosing intussusception. Previous studies have verified the high sensitivity (97.9%ŌĆō98.5%) and specificity (97.8%ŌĆō100%) of ultrasonography, along with high negative predictive value (99.7%) for ruling out intussusception [33,34]. The advantages of ultrasonography include a lack of radiation, easy accessibility, cost-effectiveness, and the ability to find a pathologic lead point mass or establish alternative diagnoses [30]. Typical ultrasound findings of intussusception include the target sign (transverse view) or pseudokidney sign (longitudinal view), corresponding to the intussusceptum and surrounding hyperechoic mesenteric fat within the intussuscipiens (Fig. 2) [1,30]. Several studies have reported promising results for point-of-care ultrasound (POCUS) performed by trained pediatric emergency physicians with excellent sensitivity (100%) and good specificity (94%ŌĆō95%) (Table 2) [35,36]. POCUS might be helpful in screening for suspected intussusception and ruling out nonintussusception in children early at the bedside, especially in cases of atypical or vague clinical presentation [35-39].
Computed tomography (CT) is not commonly used to diagnose intussusception because of its higher radiation dose than ultrasound. However, the use of CT can be considered for the diagnosis of intussusception in children with atypical presentation including older age, unusual site, and/or suspected pathologic lead point due to a focal mass or underlying systemic disease, such as Peutz-Jeghers syndrome [40].
Nonoperative enema reduction is the treatment of choice for childhood intussusception unless there are signs of perforation or peritonitis. Nonoperative enema reduction can be performed according to the methods of image guidance (fluoroscopy or ultrasonography) and reduction medium type (liquid or air) (Table 3). Procedural approaches depend primarily on the experience and preference of pediatric radiologists and availability of resources [41,42]. Among them, pneumatic reduction under fluoroscopic guidance is the most widely used [41]. In the past, barium was widely used as a liquid reduction medium when enema reduction was initiated; however, over time, air has become the contrast of choice for enema reduction [41,43]. One of the reasons for this is the possibility of barium staining the peritoneal cavity in cases of perforation, which makes surgical management more difficult and negatively impacts patientsŌĆÖ postoperative recovery [44]. Moreover, pneumatic reduction is clearer, less radiating, faster, and less expensive than liquid enema [30].
The basic principle of enema reduction is to move back the intussusception through the ileocecal valve by the intracolonic pressure of the reduction medium regardless of type. Before starting pneumatic enema reduction, a manometer is connected to a rectal tube to monitor the insufflation pressure. The pressure is cautiously monitored because it commonly fluctuates during insufflation and a childŌĆÖs crying. The recommended upper limit of intraluminal pressure is 120 mmHg [24]. The signs of successful enema reduction include disappearance of the soft tissue mass and rapid reflux of air into the distal small bowel (Fig. 3) [24]. A residual filling defect can indicate incomplete reduction of intussusception or a pathologic lead point but can also occur to the edematous ileocecal valve. In this case, ultrasonography was helpful in identifying the cause of a residual filling defect after pneumatic enema reduction (Fig. 4) [45]. There is no strict rule regarding the number of enema attempts, and it remains at the discretion of operators as well as clinical aspects of patients, such as age or highgrade bowel obstruction. However, the generally accepted rule is ŌĆ£3 attempts of 3 minutesŌĆØ in the same position, and successful enema reduction is less likely to be achieved if improvement does not occur after 3 separate attempts [24,29].
Hydrostatic enema reduction (commonly using saline) under ultrasonographic guidance has been increasingly used based on comparable success rates of 73%ŌĆō86% and low perforation rate (1%) by experienced providers, which has the advantage of avoiding radiation exposure [46-49]. Under ultrasonography guidance, liquid is preferred over air because of its more easily recognizable contrast to small bowel gas, which is contained at the intussusception site and interferes with sonographic visualization [29]. In this technique, saline is infused into the colon by gravity with a saline bag suspended approximately 3 feet above the table [46-48]. Successful reduction was confirmed by the disappearance of the intussusception mass and reflux of saline from the cecum to the terminal ileum through the ileocecal valve under ultrasonographic guidance.
Most small bowel intussusception cases involve a transient invagination and are likely to be reduced spontaneously, and the children can be managed with conservative observation without any intervention [50]. Follow-up ultrasonography confirms a spontaneous reduction. Nonoperative reduction is generally unsuccessful in the treatment of small bowel intussusception, and enema reduction is not recommended except in certain conditions of concomitant ileocolic or ileoileal intussusception occurring near the ileocecal valve [51]. Previous studies reported that small bowel intussusceptions involving the long segment of the bowel are associated with the need for surgical intervention [51,52]. Surgical reduction is warranted for persistent small bowel intussusception in symptomatic patients, and focal lead points are often observed in such cases [52]. In cases of colocolic intussusceptions, children can be initially managed with enema reduction as in the ileocolic type [53].
Nonoperative enema reduction is effective for children without contraindications, and a recent meta-analysis of more than 40,000 cases reported a pooled success rate of 82% [21]. Moreover, a previous meta-analysis of studies published between the 1970s and the 2010s including 32,451 children showed higher success rates with air versus liquid (barium, water, saline, or iodinated contrast material) enema (82.7% vs. 69.6%) with similar perforation rates (0.39% vs. 0.43%) [54].
A delayed repeat enema is recommended when the first attempt partially reduces the intussusception and if the patient shows stable vital signs and no signs of peritonitis, which can reduce the number of children requiring surgery [29]. The optimal time gap between enema attempts has not been established, but practitioners commonly wait for 15 minutes to a few hours [41]. The postulated mechanism is that partial reduction of the intussusception on the first attempt improves venous drainage and reduces bowel wall edema in residual intussusception [55]. The reported success rate of delayed repeat enema is approximately 50% in the children with a failed initial enema reduction [55,56].
Intussusception recurs in approximately 9%ŌĆō15% of cases after successful enema reduction [5,23,57]. Approximately one-half of recurrences are early, within the first few days after enema reduction, but it can also occur over several months or years [23,58]. Several studies have reported that older age (>1 year) at the initial presentation with intussusception is associated with an increased risk of recurrence [59-61]. Multiple recurrences are not a contraindication for nonoperative reduction, and enema reduction remains an effective primary treatment in children with hemodynamically stable and recurrent intussusception. A previous study reported a high success rate (95%) of enema reduction in 113 recurrence cases [23]. The chances of pathologic lead points increase in children with multiple recurrences (14%ŌĆō19% in more than one episode) [22,23]; therefore, a careful search for possible pathological lead points should be conducted through imaging studies. Surgical management is indicated for children with recurrent intussusception if it is irreducible by enema reduction or pathologic lead points are documented through imaging studies [23].
The rare but most important complication of enema reduction, bowel perforation, is reported in approximately 0.4% of cases of air and liquid enemas [54]. These causes are attributed to bowel wall necrosis [62] and technical factors of high enema pressure [44]. In cases of perforation during an air enema, there is a possibility of tension pneumoperitoneum and hemodynamic compromise, and emergent paracentesis should be conducted in the midline supraumbilical location [24,29,63]. Immediate surgical exploration is required to minimize peritoneal contamination by bowel contents [63].
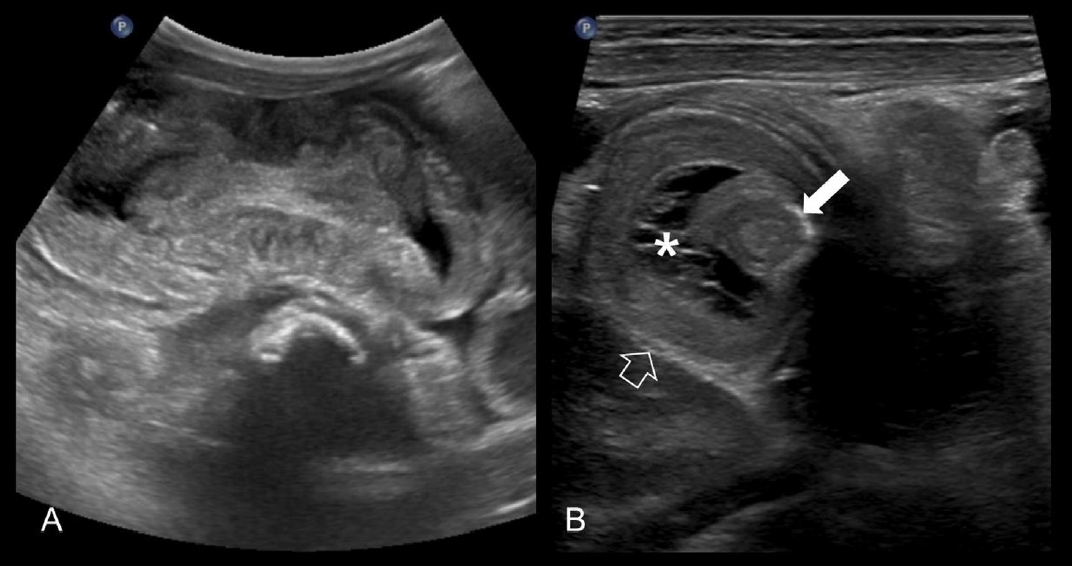
Many researchers have studied predictors of failed enema reduction. Successful enema reduction is less likely to be achieved in children with a longer symptom duration before presentation, younger age, unfavorable symptoms (lethargy, bloody diarrhea), radiologic findings (small bowel obstruction, trapped fluid between the intussusceptum and intussusceptor (Figs. 5, 6), ascites, or absence of flow in the intussusception), pathological lead points, and distant location of the intussusception (Table 4) [5,31,64-67]. A recent meta-analysis confirmed that clinical features of age <1 year, fever, rectal bleeding, and vomiting, and ultrasonographic features of ascites, left-sided intussusception, and trapped fluid between intussuscepted bowel walls were significantly associated with enema reduction failure, while a shorter symptom duration (<24 hours) and abdominal pain were associated with success [21]. Enlarged mesenteric lymph nodes and palpable masses were not significantly associated with enema reduction failure.
This study also provided a meta-regression analysis according to technical factors of enema reduction and revealed that sedation was not a significant factor of success rate heterogeneity among studies. A few studies advocate use of sedatives such as midazolam, diazepam, or propofol to reduce patient discomfort and improve success rate via smooth muscle relaxation, with reported success rates of 73%ŌĆō92% [68-70]. However, it also has disadvantages such as unpredictable response and the need for experienced medical staff who can monitor and resuscitate the patient during the procedure [71].
Sedation can also prevent the Valsalva maneuver in children, which is helpful for reduction by increasing intracolonic pressure, thereby leading to successful reduction as well as diminishing the transmural pressure gradient and protecting against perforation [72]. The risks and benefits of sedation during enema reduction remain uncertain, and its use depends on the providerŌĆÖs preference [29].
The suggested predictors of enema reduction failure are related to possible bowel ischemia but do not necessarily indicate contraindications for nonoperative enema reduction [29]. Given the high success rate and low complication (perforation) rate of nonoperative enema reduction, practitioners can attempt enema reduction as the primary initial treatment when negative predictors are present in a hemodynamically stable child but should consider predictors to avoid high-pressure enema, warning parents of the possibility of failed enema or preparing them for surgical reduction in cases of failure.
Many children with intussusception do not show a complete symptom triad, and a high level of clinical suspicion is necessary to not miss the diagnosis in children with nonspecific symptoms. The incidence of pathological lead points increases in older children with multiple episodes of recurrent intussusception. Pneumoperitoneum on radiographs, peritonitis, and shock are contraindications for nonoperative enema reduction; these children should be treated surgically. Ultrasonography is the reliable modality of choice for the diagnosis of intussusception, with high sensitivity and specificity and the ability to establish alternative diagnoses. Pneumatic reduction under fluoroscopic guidance is the most widely used and effective method of nonoperative enema reduction, with a high success rate and low perforation rate. Hydrostatic enema reduction under ultrasonographic guidance has been increasingly used because of comparable success rates and low perforation rates, with the major advantage of avoiding radiation exposure. It is helpful to recognize the predictors of enema reduction failure described in the previous literature, although providers can attempt enema reduction as the primary initial treatment in a hemodynamically stable child. A delayed repeat enema is recommended when the first attempt partially reduces the intussusception and the patient shows stable vital signs and no signs of peritonitis. Nonoperative enema reduction remains an effective primary treatment for recurrent intussusception. In cases of multiple recurrence, meticulous evaluation should be performed to identify possible pathological lead points.
Footnotes
Fig.┬Ā1.
Erect abdominal radiograph of a 22-month-old boy with a 6-hour history of cyclic abdominal pain shows a soft tissue mass (arrow) within the transverse colon due to the head of the intussusception. There are no signs of pneumoperitoneum or small bowel obstruction.
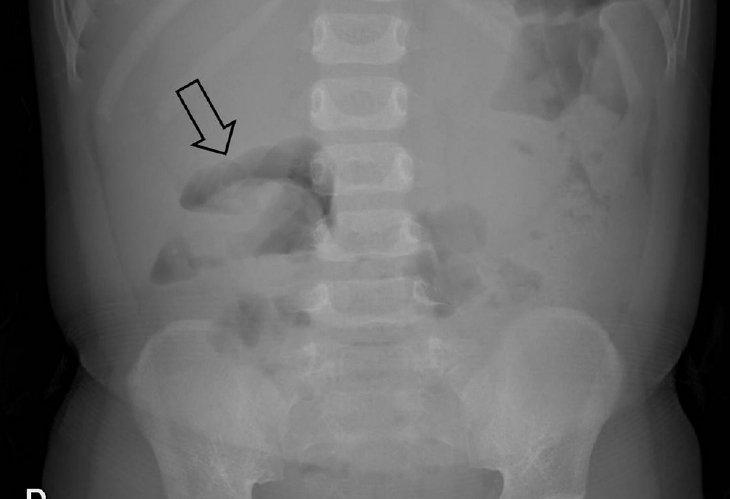
Fig.┬Ā2.
Ultrasonography images of the right upper quadrant of a 35-month-old boy with a 1-day history of cyclic abdominal pain. Transverse (A) and longitudinal (B) images show intussuscipiens (black solid arrow) containing the intussusceptum (white dashed arrow) as well as mesenteric fat and a lymph node (arrowhead). These classic appearances are called target and pseudokidney signs on the transverse and longitudinal image, respectively. Color Doppler transverse image (C) showing the same structures with intact vascularity.
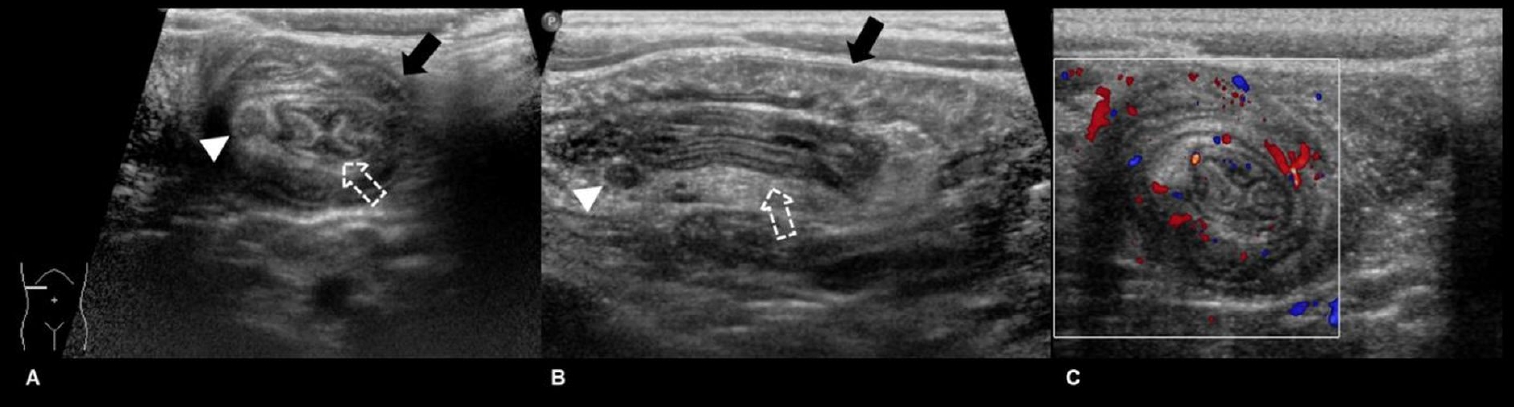
Fig.┬Ā3.
Fluoroscopy-guided air enema reduction of ileocolic intussusception in a 4-year-old boy with a 1-day history of abdominal pain. (A) Fluoroscopic spot film showing a filling defect (arrow) within the hepatic flexure of colon caused by the head of the intussusception. (B) The intussusception (arrow) is reduced back to the ileocecal valve. (C) Disappearance of the soft tissue mass and reflux of air into the distal small bowel indicates successful enema reduction.
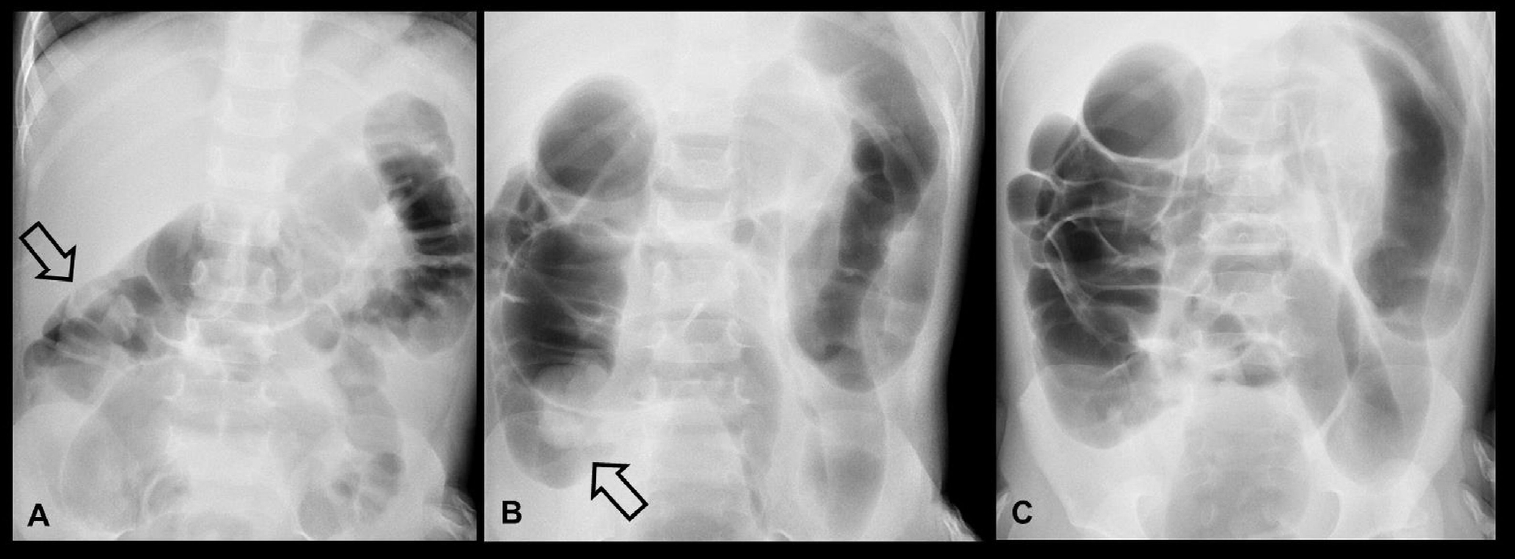
Fig.┬Ā4.
(A) After fluoroscopy-guided air enema reduction of ileocolic intussusception in a 10-year-old boy, a residual filling defect is shown in the right lower quadrant (arrow). Ultrasonography was subsequently performed to differetiate between incomplete reduction, a pathologic lead point, and pseudomass due to edematous ileocecal valve. Ultrasonography revealed a hypoechoic polypoid mass in the cecum (B) with increased internal vascularity (C). The mass was surgically confirmed as diffuse large B cell lymphoma.
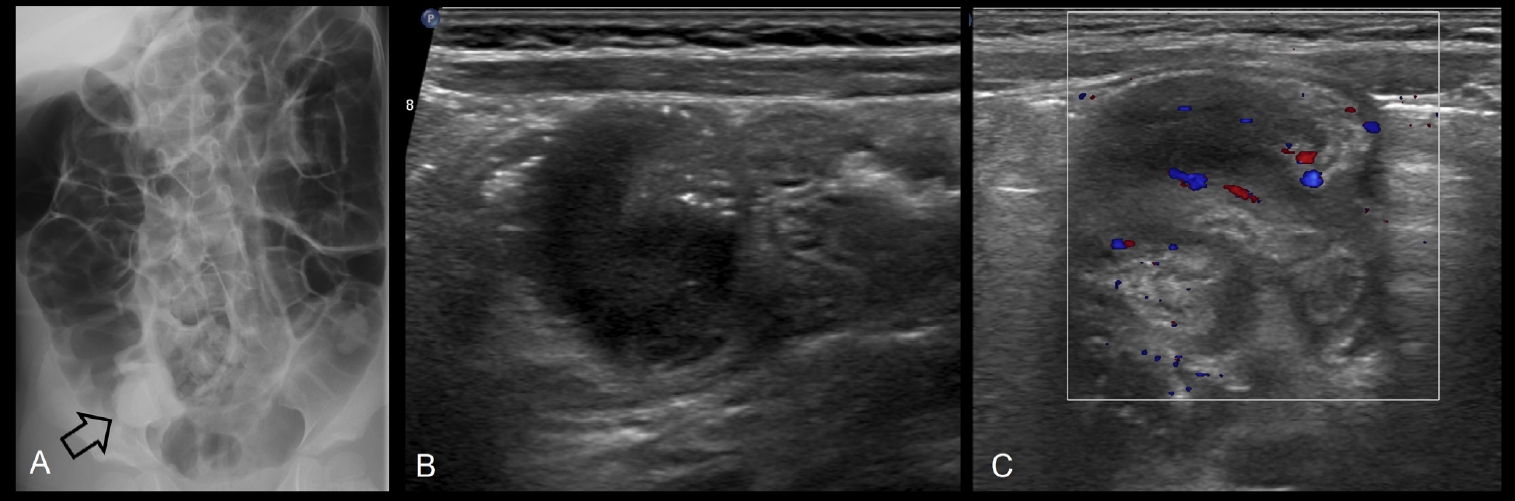
Fig.┬Ā5.
Erect abdominal radiograph of a 11-month-old boy showing gaseous dilatation of the small bowel with multiple air-fluid levels, indicating small bowel obstruction (A). He was diagnosed with ileocolic intussusception by ultrasonography (B) and small bowel retention (asterisks) with thickening of the bowel wall (C). (D) Subsequent pneumatic enema reduction was successful and fluoroscopy spot film showed disappearance of the soft tissue mass, reflux of air into the distal small bowel, and a swollen ileocecal valve (arrow). (E) However, an abdominal radiograph taken on the 3rd day of admission revealed pneumoperitoneum (arrows) and emergent laparotomy confirmed perforation in transverse colon due to ischemic change.
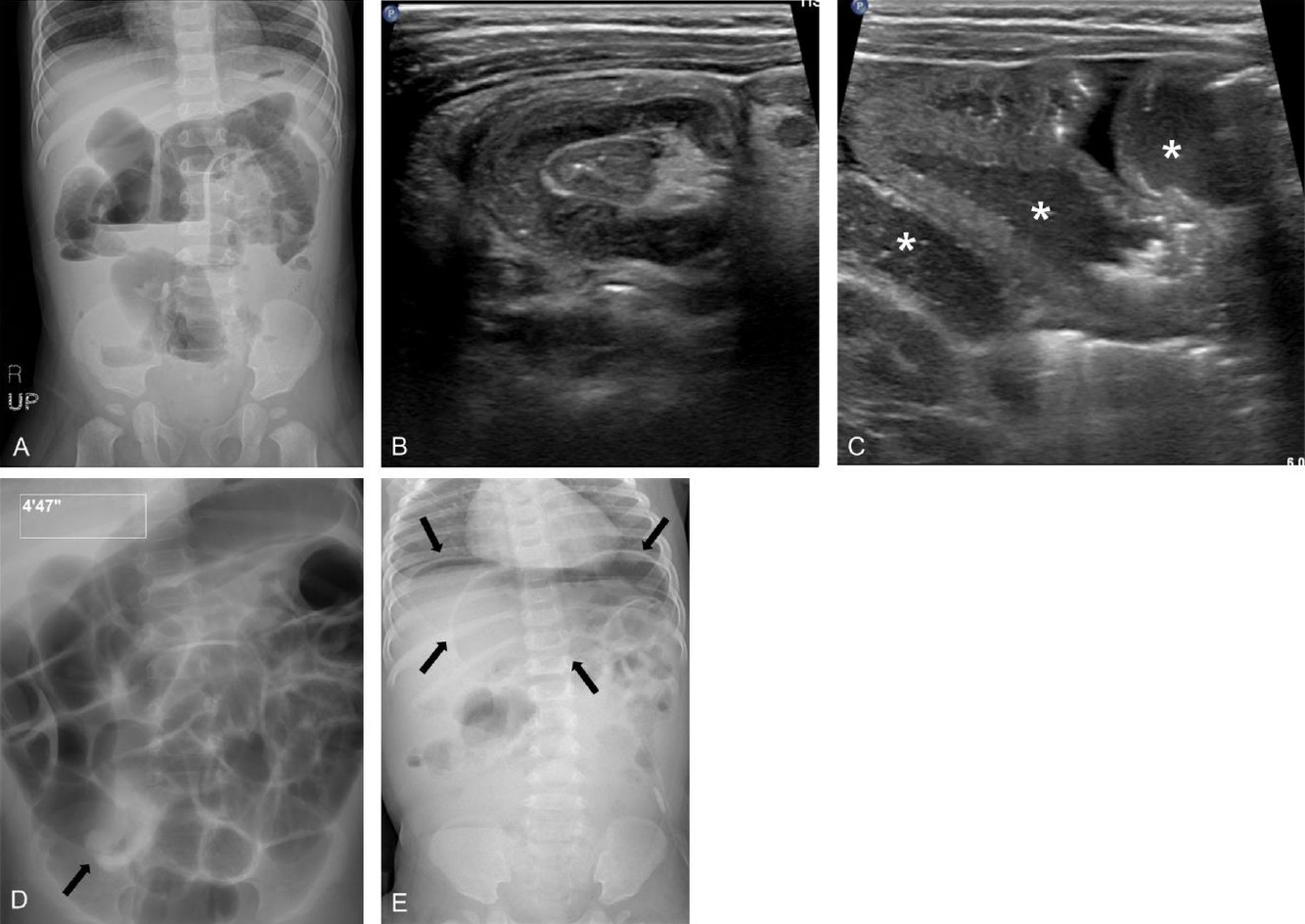
Fig.┬Ā6.
(A) Longitudinal ultrasonography images of a 11-month-old boy showing ileocolic intussusception with pseudokidney signs. (B) On a transverse image, trapped fluid (asterisk) is seen between the intussusceptum (solid arrow) and the intussuscipien (open arrow).
Table┬Ā1.
Summary of epidemiology of intussusception
Table┬Ā2.
Newer approaches to imaging diagnosis of intussusception
| Methods | Outcome | Advantage | Future research directions |
|---|---|---|---|
| Point-of-care ultrasound (POCUS) [36,38,39,73] | POCUS is highly sensitive (94.9%) and specific (99.1%) for the detection of intussusception for children presenting to the emergency department (ED). | POCUS could streamline the ED workflow of clinically low risk of intussusception and unnecessary referrals for ultrasound. | Establishment of the optimal training curriculum; standardized ultrasound scanning protocols to minimize variation; usefulness of POCUS in correlation with clinical outcome; cost-effectiveness of POCUS |
| Implementation of POCUS for clinically nonspecific intussusception leaded to a shorter median ED length of stay, door-to-reduction time, and observation time. | |||
| Deep learningbased algorithms using abdominal radiographs [27,28] | The sensitivity of the YOLOv3-based algorithm was higher compared with that of the radiologists (0.76 vs. 0.46), while specificity was not different (0.96 vs. 0.92). | Deep learning could help screen children who need US or referral to other hospitals without being influenced by the level of experience in pediatric radiographs. | Further external validation with large datasets and multiple institutions to explore clinical outcome; technical improvement in deep learning |
| The external test values were 0.811 to 0.895 for the area under the receiver operating characteristic curve (sensitivity, 0.651ŌĆō0.947; specificity, 0.485ŌĆō0.842). |
Table┬Ā3.
Table┬Ā4.
References
1. Edwards EA, Pigg N, Courtier J, Zapala MA, MacKenzie JD, Phelps AS. Intussusception: past, present and future. Pediatr Radiol 2017;47:1101ŌĆō8.



2. Buettcher M, Baer G, Bonhoeffer J, Schaad UB, Heininger U. Three-year surveillance of intussusception in children in Switzerland. Pediatrics 2007;120:473ŌĆō80.



3. Jo S, Lim IS, Chae SA, Yun SW, Lee NM, Kim SY, et al. Characteristics of intussusception among children in Korea: a nationwide epidemiological study. BMC Pediatrics 2019;19:211.




4. Mandeville K, Chien M, Willyerd FA, Mandell G, Hostetler MA, Bulloch B. Intussusception: clinical presentations and imaging characteristics. Pediatr Emerg Care 2012;28:842ŌĆō4.

5. Lee EH, Yang HR. Nationwide population-based epidemiologic study on childhood intussusception in South Korea: emphasis on treatment and outcomes. Pediatr Gastroenterol Hepatol Nutr 2020;23:329ŌĆō45.




7. Kaiser AD, Applegate KE, Ladd AP. Current success in the treatment of intussusception in children. Surgery 2007;142:469. ŌĆō75. ; discussion 475-7.


8. Mart├Łnez Biarge M, Garc├Ła-Alix A, Luisa del Hoyo M, Alarc├│n A, S├Īenz de Pipa├│n M, Hern├Īndez F, et al. Intussusception in a preterm neonate; a very rare, major intestinal problem--systematic review of cases. J Perinat Med 2004;32:190ŌĆō4.

9. Guo WL, Zhang SF, Li JE, Wang J. Association of meteorological factors with pediatric intussusception in subtropical china: a 5-year analysis. PLoS One 2014;9:e90521.



10. Samad L, Cortina-Borja M, Bashir HE, Sutcliffe AG, Marven S, Cameron JC, et al. Intussusception incidence among infants in the UK and Republic of Ireland: a pre-rotavirus vaccine prospective surveillance study. Vaccine 2013;31:4098ŌĆō102.



11. Nelson EA, Tam JS, Glass RI, Parashar UD, Fok TF. Incidence of rotavirus diarrhea and intussusception in Hong Kong using standardized hospital discharge data. Pediatr Infect Dis J 2002;21:701ŌĆō3.


12. Liu N, Yen C, Huang T, Cui P, Tate JE, Jiang B, et al. Incidence and epidemiology of intussusception among children under 2years of age in Chenzhou and Kaifeng, China, 2009-2013. Vaccine 2018;36:7862ŌĆō7.



13. Muhsen K, Kassem E, Efraim S, Goren S, Cohen D, Ephros M. Incidence and risk factors for intussusception among children in northern Israel from 1992 to 2009: a retrospective study. BMC Pediatrics 2014;14:218.




14. Mansour AM, El Koutby M, El Barbary MM, Mohamed W, Shehata S, El Mohammady H, et al. Enteric viral infections as potential risk factors for intussusception. J Infect Dev Ctries 2013;7:28ŌĆō35.



15. Nylund CM, Denson LA, Noel JM. Bacterial enteritis as a risk factor for childhood intussusception: a retrospective cohort study. J Pediatr 2010;156:761ŌĆō5.


16. Hsu HY, Kao CL, Huang LM, Ni YH, Lai HS, Lin FY, et al. Viral etiology of intussusception in Taiwanese childhood. Pediatr Infect Dis J 1998;17:893ŌĆō8.


17. Bines JE, Liem NT, Justice FA, Son TN, Kirkwood CD, de Campo M, et al. Risk factors for intussusception in infants in Vietnam and Australia: adenovirus implicated, but not rotavirus. J Pediatr 2006;149:452ŌĆō60.


18. Okimoto S, Hyodo S, Yamamoto M, Nakamura K, Kobayashi M. Association of viral isolates from stool samples with intussusception in children. Int J Infect Dis 2011;15:e641ŌĆō5.


19. Yoo IH, Kang HM, Jeong DC. Changes in the incidence of intussusception and infectious diseases after the COVID-19 pandemic in Korea. J Korean Med Sci 2022;37:e60.




20. Fiegel H, Gfroerer S, Rolle U. Systematic review shows that pathological lead points are important and frequent in intussusception and are not limited to infants. Acta Paediatr 2016;105:1275ŌĆō9.



21. Kim PH, Hwang J, Yoon HM, Lee JY, Jung AY, Lee JS, et al. Predictors of failed enema reduction in children with intussusception: a systematic review and meta-analysis. Eur Radiol 2021;31:8081ŌĆō97.



22. Navarro O, Daneman A. Intussusception. Part 3: Diagnosis and management of those with an identifiable or predisposing cause and those that reduce spontaneously. Pediatr Radiol 2004;34:305. ŌĆō12. quiz 369.

23. Daneman A, Alton DJ, Lobo E, Gravett J, Kim P, Ein SH. Patterns of recurrence of intussusception in children: a 17-year review. Pediatr Radiol 1998;28:913ŌĆō9.



24. American College of Radiology. ACR-SPR practice parameter for the performance of pediatric fluoroscopic contrast enema examinations [Internet]. Reston (VA): American College of Radiology; [revised 2021; cited 2021 Oct 17]. Available from: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/FluourConEnema-Ped.pdf.
25. Hooker RL, Hernanz-Schulman M, Yu C, Kan JH. Radiographic evaluation of intussusception: utility of left-side-down decubitus view. Radiology 2008;248:987ŌĆō94.



26. Sargent MA, Babyn P, Alton DJ. Plain abdominal radiography in suspected intussusception: a reassessment. Pediatr Radiol 1994;24:17ŌĆō20.



27. Kim S, Yoon H, Lee MJ, Kim MJ, Han K, Yoon JK, et al. Performance of deep learning-based algorithm for detection of ileocolic intussusception on abdominal radiographs of young children. Sci Rep 2019;9:19420.




28. Kwon G, Ryu J, Oh J, Lim J, Kang BK, Ahn C, et al. Deep learning algorithms for detecting and visualising intussusception on plain abdominal radiography in children: a retrospective multicenter study. Sci Rep 2020;10:17582.




29. Ito Y, Kusakawa I, Murata Y, Ukiyama E, Kawase H, Kamagata S, et al. Japanese guidelines for the management of intussusception in children, 2011. Pediatr Int 2012;54:948ŌĆō58.


30. Applegate KE. Intussusception in children: evidence-based diagnosis and treatment. Pediatr Radiol 2009;39 Suppl 2:S140ŌĆō3.



31. McDermott VG, Taylor T, Mackenzie S, Hendry GM. Pneumatic reduction of intussusception: clinical experience and factors affecting outcome. Clin Radiol 1994;49:30ŌĆō4.


32. Fike FB, Mortellaro VE, Holcomb GW 3rd, St Peter SD. Predictors of failed enema reduction in childhood intussusception. J Pediatr Surg 2012;47:925ŌĆō7.


33. Hryhorczuk AL, Strouse PJ. Validation of US as a first-line diagnostic test for assessment of pediatric ileocolic intussusception. Pediatr Radiol 2009;39:1075ŌĆō9.



34. Shanbhogue RL, Hussain SM, Meradji M, Robben SG, Vernooij JE, Molenaar JC. Ultrasonography is accurate enough for the diagnosis of intussusception. J Pediatr Surg 1994;29:324. ŌĆō7. discussion 327-8.


35. Lam SH, Wise A, Yenter C. Emergency bedside ultrasound for the diagnosis of pediatric intussusception: a retrospective review. World J Emerg Med 2014;5:255ŌĆō8.



36. Lee JY, Kim JH, Choi SJ, Lee JS, Ryu JM. Point-of-care ultrasound may be useful for detecting pediatric intussusception at an early stage. BMC Pediatrics 2020;20:155.




37. Han SM, Kim JH, Lee JS. Point-of-care ultrasound may reduce emergency department length of stay in children with nonspecific manifestations of intussusception. Pediatr Emerg Med J 2016;3:15ŌĆō23.


38. Lin-Martore M, Kornblith AE, Kohn MA, Gottlieb M. Diagnostic accuracy of point-of-care ultrasound for intussusception in children presenting to the emergency department: a systematic review and metaanalysis. West J Emerg Med 2020;21:1008ŌĆō16.



39. Kim JH, Lee JY, Kwon JH, Cho HR, Lee JS, Ryu JM. Point-of-care ultrasound could streamline the emergency department workflow of clinically nonspecific intussusception. Pediatr Emerg Care 2020;36:e90ŌĆō5.


40. Cox TD, Winters WD, Weinberger E. CT of intussusception in the pediatric patient: diagnosis and pitfalls. Pediatr Radiol 1996;26:26ŌĆō32.



41. Stein-Wexler R, O'Connor R, Daldrup-Link H, Wootton-Gorges SL. Current methods for reducing intussusception: survey results. Pediatr Radiol 2015;45:667ŌĆō74.



42. Ogundoyin O, Lawal T, Olulana D, Atalabi O. Experience with sonogramguided hydrostatic reduction of intussusception in children in South-West Nigeria. J West Afr Coll Surg 2013;3:76ŌĆō88.


43. Yoon CH, Kim HJ, Goo HW. Recent trends of radiological reduction of intussusception in children: a nationwide phone survey to training hospitals in Korea. J Korean Radiol Soc 2000;43:765ŌĆō9.

44. Daneman A, Alton DJ, Ein S, Wesson D, Superina R, Thorner P. Perforation during attempted intussusception reduction in children--a comparison of perforation with barium and air. Pediatr Radiol 1995;25:81ŌĆō8.



45. Kitazono MT, Pollock AN. Intussusception: edematous ileocecal valve mimicking incomplete reduction. Pediatr Emerg Care 2012;28:300ŌĆō1.

46. Flaum V, Schneider A, Gomes Ferreira C, Philippe P, Sebastia Sancho C, Lacreuse I, et al. Twenty years' experience for reduction of ileocolic intussusceptions by saline enema under sonography control. J Pediatr Surg 2016;51:179ŌĆō82.


47. Gondek AS, Riaza L, Cuadras D, Castellarnau XT, Krauel L. Ileocolic intussusception: predicting the probability of success of ultrasound guided saline enema from clinical and sonographic data. J Pediatr Surg 2018;53:599ŌĆō604.


48. Menke J, Kahl F. Sonography-guided hydrostatic reduction of ileocolic intussusception in children: analysis of failure and success in consecutive patients presenting timely to the hospital. Eur J Pediatr 2015;174:307ŌĆō16.



49. Plut D, Phillips GS, Johnston PR, Lee EY. Practical imaging strategies for intussusception in children. Am J Roentgenol 2020;215:1449ŌĆō63.

50. Doi O, Aoyama K, Hutson JM. Twenty-one cases of small bowel intussusception: the pathophysiology of idiopathic intussusception and the concept of benign small bowel intussusception. Pediatr Surg Int 2004;20:140ŌĆō3.



51. Kim JH. US features of transient small bowel intussusception in pediatric patients. Korean J Radiol 2004;5:178ŌĆō84.



52. Munden MM, Bruzzi JF, Coley BD, Munden RF. Sonography of pediatric small-bowel intussusception: differentiating surgical from nonsurgical cases. Am J Roentgenol 2007;188:275ŌĆō9.

53. Richer EJ, Dickson PN. Colocolic intussusceptions in children: a pictorial essay and review of the literature. Emerg Radiol 2020;27:97ŌĆō102.



54. Sadigh G, Zou KH, Razavi SA, Khan R, Applegate KE. Meta-analysis of air versus liquid enema for intussusception reduction in children. Am J Roentgenol 2015;205:W542ŌĆō9.

55. Saxton V, Katz M, Phelan E, Beasley SW. Intussusception: a repeat delayed gas enema increases the nonoperative reduction rate. J Pediatr Surg 1994;29:588ŌĆō9.


56. Navarro OM, Daneman A, Chae A. Intussusception: the use of delayed, repeated reduction attempts and the management of intussusceptions due to pathologic lead points in pediatric patients. Am J Roentgenol 2004;182:1169ŌĆō76.
57. Gray MP, Li SH, Hoffmann RG, Gorelick MH. Recurrence rates after intussusception enema reduction: a meta-analysis. Pediatrics 2014;134:110ŌĆō9.



58. Whitehouse JS, Gourlay DM, Winthrop AL, Cassidy LD, Arca MJ. Is it safe to discharge intussusception patients after successful hydrostatic reduction? J Pediatr Surg 2010;45:1182ŌĆō6.


59. Guo WL, Hu ZC, Tan YL, Sheng M, Wang J. Risk factors for recurrent intussusception in children: a retrospective cohort study. BMJ Open 2017;7:e018604.



60. Lee DH, Kim SJ, Lee HJ, Jang HJ. Identifying predictive factors for the recurrence of pediatric intussusception. Pediatr Gastroenterol Hepatol Nutr 2019;22:142ŌĆō51.




61. Wang Z, He QM, Zhang H, Zhong W, Xiao WQ, Lu LW, et al. Intussusception patients older than 1 year tend to have early recurrence after pneumatic enema reduction. Pediatr Surg Int 2015;31:855ŌĆō8.



62. Bramson RT, Blickman JG. Perforation during hydrostatic reduction of intussusception: proposed mechanism and review of the literature. J Pediatr Surg 1992;27:589ŌĆō91.


63. Fallon SC, Kim ES, Naik-Mathuria BJ, Nuchtern JG, Cassady CI, Rodriguez JR. Needle decompression to avoid tension pneumoperitoneum and hemodynamic compromise after pneumatic reduction of pediatric intussusception. Pediatr Radiol 2013;43:662ŌĆō7.



64. Chung JL, Kong MS, Lin JN, Wang KL, Lou CC, Wong HF. Intussusception in infants and children: risk factors leading to surgical reduction. J Formos Med Assoc 1994;93:481ŌĆō5.

65. Ntoulia A, Tharakan SJ, Reid JR, Mahboubi S. Failed intussusception reduction in children: correlation between radiologic, surgical, and pathologic findings. Am J Roentgenol 2016;207:424ŌĆō33.

66. Lim HK, Bae SH, Lee KH, Seo GS, Yoon GS. Assessment of reducibility of ileocolic intussusception in children: usefulness of color Doppler sonography. Radiology 1994;191:781ŌĆō5.


67. Khorana J, Singhavejsakul J, Ukarapol N, Laohapensang M, Siriwongmongkol J, Patumanond J. Prognostic indicators for failed nonsurgical reduction of intussusception. Ther Clin Risk Manag 2016;12:1231ŌĆō7.



68. Sacks RS, Anconina R, Farkas E, Zolotnik-Krupenich D, Kravarusic D, Tsodikov V, et al. Sedated ultrasound guided saline reduction (SUR) of ileocolic intussusception: 20year experience. J Pediatr Surg 2020;55:2009ŌĆō14.


69. Ilivitzki A, Shtark LG, Arish K, Engel A. Deep sedation during pneumatic reduction of intussusception. Pediatr Radiol 2012;42:562ŌĆō5.



70. Chan KL, Saing H, Peh WC, Mya GH, Cheng W, Khong PL, et al. Childhood intussusception: ultrasound-guided HartmannŌĆÖs solution hydrostatic reduction or barium enema reduction? J Pediatr Surg 1997;32:3ŌĆō6.


71. Daneman A, Navarro O. Intussusception. Part 2: an update on the evolution of management. Pediatr Radiol 2004;34:97. ŌĆō108. quiz 187.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


