Article Contents
| Clin Exp Pediatr > Volume 66(10); 2023 |
|
Abstract
Supplementary materials
Supplementary Table 1.
Supplementary Fig. 1.
Footnotes
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: Lee YK, Kwon Y, Kim EK and Cho E; Data curation: Seo SY, Kim SY, Kim S, Ko M and Cho H; Formal analysis:Kwon Y, Heo Y, Kim SY, and Kim S; Methodology: Kwon Y, Kim EK, Seo SY, Kim SY, Lee YK, and Cho H; Project administration: Kwon Y, Lee YK, and Cho E; Visualization: Kim S, Ko M and Heo Y; Writing - original draft: Lee YK, Kwon Y, and Kim EK; Writing - review & editing: Lee YK, Kwon Y, Heo Y, Kim EK, Kim SY, Cho H, Kim S, Ko M, Lim D, Seo SY, Cho E
Acknowledgments
Fig. 1.
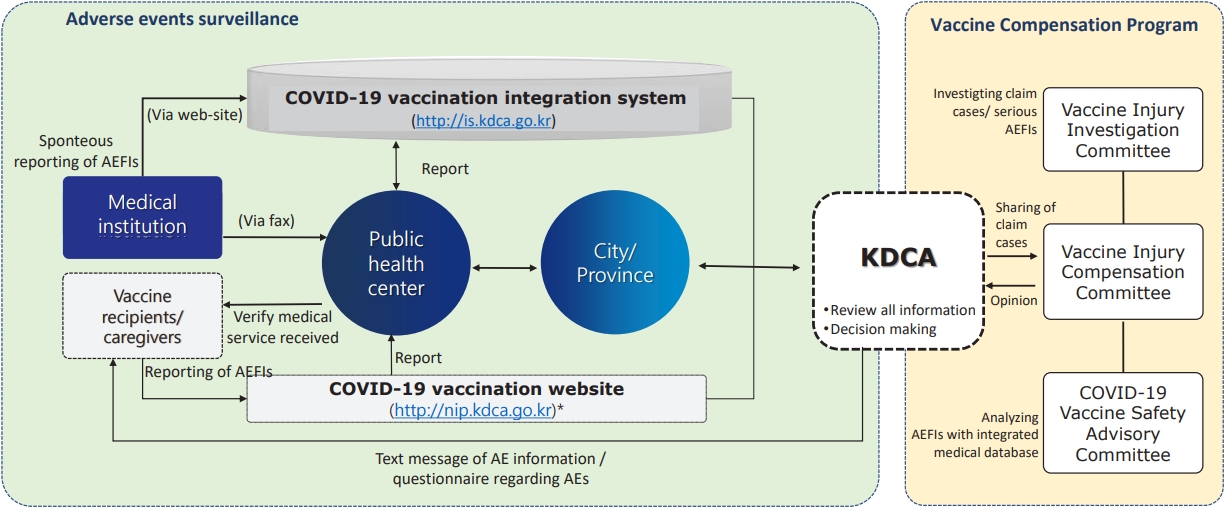
Fig. 2.
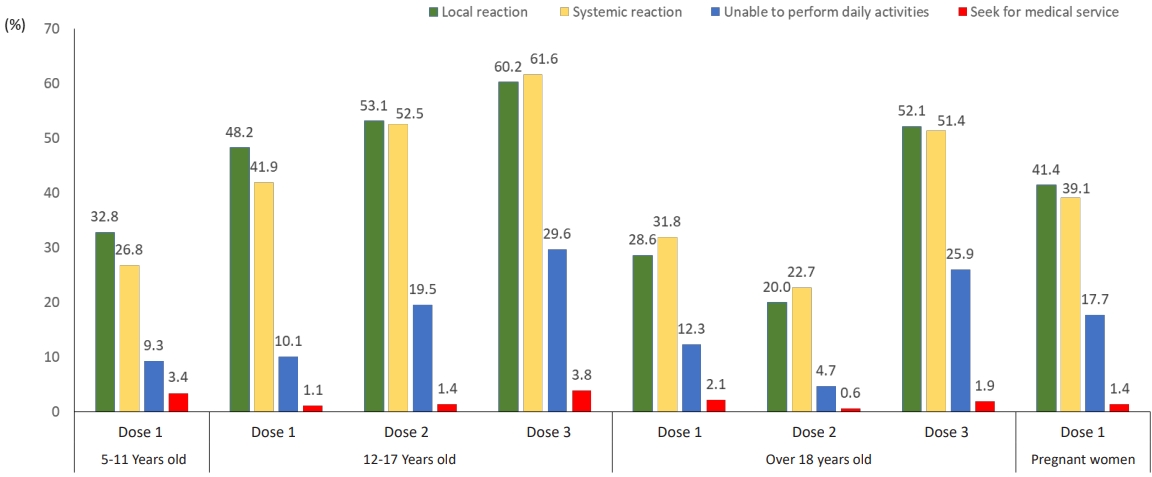
Table 1.
| Variable | Dose administereda) |
Reported AE cases after COVID-19 vaccinationb) |
|||||
|---|---|---|---|---|---|---|---|
| Frequency | Nonserious Aesc) | Serious Aesd) | Death | ||||
| Total | 125,107,883 | 471,068 (376.5) | 452,530 (361.7) | 18,538 (14.8) | 1,654 | ||
| Sex | |||||||
| 5–11 Years old | 90,580 | 73 (80.6) | 70 (77.3) | 3 (3.3) | 0 | ||
| Male | 46,186 | 44 (95.3) | 43 (93.1) | 1 (2.2) | 0 | ||
| Female | 44,394 | 29 (65.3) | 27 (60.8) | 2 (4.5) | 0 | ||
| 12–17 Years old | 5,315,096 | 15,777 (296.8) | 15,316 (288.2) | 461 (8.7) | 5 | ||
| Male | 2,741,950 | 7,802 (284.5) | 7,538 (274.9) | 264 (9.6) | 3 | ||
| Female | 2,573,146 | 7,975 (309.9) | 7,778 (302.3) | 197 (7.7) | 2 | ||
| Over 18 years old | 119,702,207 | 455,218 (380.3) | 437,144 (365.2) | 18,074 (15.1) | 1,649 | ||
| Male | 59,118,024 | 161,837 (273.8) | 153,542 (259.7) | 8,295 (14.0) | 1,051 | ||
| Female | 60,584,183 | 293,381 (484.3) | 283,602 (468.1) | 9,779 (16.1) | 598 | ||
| Age group (yr) | |||||||
| 5–11 | 90,580 | 73 (80.6) | 70 (77.3) | 3 (3.3) | 0 | ||
| 12–17 | 5,315,096 | 15,777 (296.8) | 15,316 (288.2) | 461 (8.7) | 5 | ||
| 18–19 | 2,543,855 | 10,329 (406.0) | 10,038 (394.6) | 291 (11.4) | 5 | ||
| 20–29 | 16,922,337 | 80,855 (477.8) | 78,910 (466.3) | 1,945 (11.5) | 40 | ||
| 30–39 | 15,815,568 | 77,630 (490.8) | 75,388 (476.7) | 2,242 (14.2) | 78 | ||
| 40–49 | 20,794,325 | 79,321 (381.5) | 76,781 (369.2) | 2,540 (12.2) | 128 | ||
| 50–59 | 23,960,610 | 81,964 (342.1) | 78,995 (329.7) | 2,969 (12.4) | 241 | ||
| 60–69 | 21,389,396 | 78,299 (366.1) | 74,279 (347.3) | 4,020 (18.8) | 371 | ||
| 70–79 | 12,018,960 | 34,262 (285.1) | 31,721 (263.9) | 2,541 (21.1) | 355 | ||
| ≥80 | 6,257,156 | 12,558 (200.7) | 11,032 (176.3) | 1,526 (24.4) | 431 | ||
| Dose | |||||||
| Dose 1 | 44,819,538 | 239,933 (535.3) | 229,629 (512.3) | 10,304 (23.0) | 599 | ||
| Dose 2 | 42,862,748 | 174,710 (407.6) | 168,899 (394.0) | 5,811 (13.6) | 604 | ||
| Dose 3 | 33,248,513 | 54,140 (162.8) | 51,858 (156.0) | 2,282 (6.9) | 414 | ||
| Dose 4 | 4,177,084 | 2,285 (54.7) | 2,144 (51.3) | 141 (3.4) | 37 | ||
| Vaccine brande) | |||||||
| AstraZeneca | 20,348,861 | 109,809 (539.6) | 104,110 (511.6) | 5,699 (28.0) | 444 | ||
| Pfizer | 78,128,712 | 240,126 (307.3) | 230,283 (294.7) | 9,843 (12.6) | 949 | ||
| Moderna | 24,581,600 | 111,471 (453.5) | 108,917 (443.1) | 2,554 (10.4) | 239 | ||
| Janssen | 1,509,197 | 8,855 (586.7) | 8,451 (560.0) | 404 (26.8) | 14 | ||
| Novavax | 539,513 | 807 (149.6) | 769 (142.5) | 38 (7.0) | 8 | ||
Units: n, frequency per 100,000 doses.
COVID-19, coronavirus disease 2019; ICU, intensive care unit.
AstraZeneca, AstraZeneca COVID-19 vaccine (ChAdOx1-S); Janssen, Janssen COVID-19 vaccine (Ad26.COV2.S); Pfizer, Pfizer-BioNTech COVID-19 vaccine (BNT162b2); Moderna, Moderna COVID-19 Vaccine (mRNA-1273); Novavax, Nuvaxovid prefilled syringe COVID-19 vaccine (NVX-CoV2373).
b) These cases reported as suspected AEs following COVID-19 vaccination were calculated based on the information reported by medical institutions and did not indicate causality between vaccines and AEs. The report status classification can change with the addition of new information.
c) Nonserious adverse events include symptoms frequently soliciting after vaccination, such as local injection site reactions (redness, pain, and swelling), muscle pain, fever, headache, and chills.
Table 2.
| Doses administereda) |
Reported AEs cases after booster vaccinationb) |
|||||
|---|---|---|---|---|---|---|
| Cases | Nonserious Aesc) | Serious Aesd) | Death | |||
| Total | 33,248,513 | 54,140 (162.8) | 51,858 (156.0) | 2,282 (6.9) | 414 | |
| 12–17 Years old | 415,590e) | 1,056 (254.1) | 1,042 (250.7) | 14 (3.4) | 0 | |
| Male | 221,642 | 556 (250.9) | 546 (246.3) | 10 (4.5) | 0 | |
| Female | 193,948 | 500 (257.8) | 496 (255.7) | 4 (2.1) | 0 | |
| Over 18 years old | 32,832,923 | 53,084 (161.7) | 50,816 (154.8) | 2,268 (6.9) | 414 | |
| Male | 16,319,973 | 21,033 (128.9) | 19,813 (121.4) | 1,220 (7.5) | 262 | |
| Female | 16,512,950 | 32,051 (194.1) | 31,003 (187.7) | 1,048 (6.3) | 152 | |
| Age group (yr) | ||||||
| 12–17 | 415,590 | 1,056 (254.1) | 1,042 (250.7) | 14 (3.4) | 0 | |
| 18–19 | 719,363 | 1,678 (233.3) | 1,632 (226.9) | 46 (6.4) | 0 | |
| 20–29 | 3,886,946 | 7,795 (200.5) | 7,662 (197.1) | 133 (3.4) | 6 | |
| 30–39 | 3,965,575 | 7,618 (192.1) | 7,417 (187.0) | 201 (5.1) | 12 | |
| 40–49 | 5,534,489 | 8,628 (155.9) | 8,348 (150.8) | 280 (5.1) | 28 | |
| 50–59 | 7,145,250 | 9,665 (135.3) | 9,274 (129.8) | 391 (5.5) | 55 | |
| 60–69 | 6,441,346 | 10,238 (158.9) | 9,689 (150.4) | 549 (8.5) | 101 | |
| 70–79 | 3,403,634 | 5,255 (154.4) | 4,847 (142.4) | 408 (12.0) | 107 | |
| ≥80 | 1,736,320 | 2,207 (127.1) | 1,947 (112.1) | 260 (15.0) | 105 | |
| Vaccine brand | ||||||
| AstraZeneca | 135 | 1 (740.7) | 0 (0) | 1 (740.7) | 0 | |
| Pfizer | 22,305,846 | 36,270 (162.6) | 34,732 (155.7) | 1,538 (6.9) | 287 | |
| Moderna | 10,849,608 | 17,703 (163.2) | 16,968 (156.4) | 735 (6.8) | 125 | |
| Janssen | 26,339 | 56 (212.6) | 52 (197.4) | 4 (15.2) | 1 | |
| Novavax | 66,585 | 110 (165.2) | 106 (159.2) | 4 (6.0) | 1 | |
Units: n (cases per 100,000 doses).
COVID-19, coronavirus disease 2019.
AstraZeneca, AstraZeneca COVID-19 vaccine (ChAdOx1-S); Janssen, Janssen COVID-19 vaccine (Ad26.COV2.S); Pfizer, Pfizer-BioNTech COVID-19 vaccine (BNT162b2); Moderna, Moderna COVID-19 Vaccine (mRNA-1273); Novavax, Nuvaxovid prefilled syringe COVID-19 vaccine (NVX-CoV2373)
b) These cases reported as suspected AEs following COVID-19 vaccination were calculated based on information reported by medical institutions and did not indicate causality between vaccines and AEs. The report status classification can change with the addition of new information.
c) Nonserious adverse events include symptoms frequently soliciting after vaccination such as injection site reactions (redness, pain, swelling, etc.), myalgia, fever, headache, chills, etc.
Table 3.
Unit: n (cases per million doses).
The Brighton collaboration case definition uses a combination of symptoms to define diagnostic accuracy levels. Levels 1–3 represent the highest diagnostic accuracy of anaphylaxis (with level 1 > level 2 > level 3); level 4 is a case reported as anaphylaxis with insufficient evidence to meet any diagnostic accuracy level; and level 5 is a case that did not meet the case definition (not a case of anaphylaxis).20) This study included level 1, 2, and 3 anaphylaxis cases.
AstraZeneca, AstraZeneca COVID-19 vaccine (ChAdOx1-S); Janssen, Janssen COVID-19 vaccine (Ad26.COV2.S); Pfizer, Pfizer-BioNTech COVID-19 vaccine (BNT162b2); Moderna, Moderna COVID-19 Vaccine (mRNA-1273); Novavax, Nuvaxovid prefilled syringe COVID-19 vaccine (NVX-CoV2373).
Table 4.
| Characteristic |
Confirmed myocarditis |
Confirmed pericarditis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total |
Dose |
Sex |
Total |
Dose |
Sex |
||||||||
| Dose 1 | Dose 2 | Dose 3&4 | M | F | Dose 1 | Dose 2 | Dose 3&4 | M | F | ||||
| Total | 511 (4.1) | 193 (4.3) | 237 (5.5) | 81 (2.2) | 323 (5.2) | 188 (3.0) | 210 (1.7) | 88 (2.0) | 97 (2.3) | 25 (0.7) | 121 (2.0) | 89 (1.4) | |
| Pfizer | 342 (4.4) | 124 (4.9) | 160 (5.9) | 58 (2.2) | 217 (5.8) | 125 (3.1) | 143 (1.8) | 53 (2.1) | 70 (2.6) | 20 (0.8) | 82 (2.2) | 61 (1.5) | |
| ≤19 yr | 132 (17.3) | 36 (10.8) | 82 (25.7) | 14 (12.9) | 110 (27.9) | 22 (6) | 28 (3.7) | 7 (2.1) | 16 (5) | 5 (4.6) | 19 (4.8) | 9 (2.4) | |
| 20–29 yr | 69 (5.3) | 29 (5.9) | 23 (4.6) | 17a) (5.3) | 45 (6.6) | 24 (3.9) | 37 (2.8) | 14 (2.9) | 19 (3.8) | 4 (1.2) | 21 (3.1) | 16 (2.6) | |
| 30–39 yr | 46 (4.5) | 29 (8.1) | 16 (4) | 1 (0.4) | 24 (5.1) | 22 (4) | 33 (3.2) | 16 (4.5) | 15 (3.8) | 2 (0.8) | 15 (3.2) | 18 (3.3) | |
| 40–49 yr | 39 (2.7) | 14 (2.9) | 18 (3.3) | 7 (1.6) | 16 (2.2) | 23 (3.1) | 21 (1.4) | 7 (1.5) | 9 (1.6) | 5 (1.2) | 10 (1.4) | 11 (1.5) | |
| 50–59 yr | 39 (2.5) | 15 (3) | 16 (3) | 8 (1.6) | 13 (1.7) | 26 (3.3) | 17 (1.1) | 9 (1.8) | 7 (1.3) | 1 (0.2) | 13 (1.7) | 4 (0.5) | |
| ≥60 yr | 17 (1) | 1 (0.3) | 5 (1.3) | 11 (1.2) | 9 (1.3) | 8 (0.8) | 7 (0.4) | 0 (0) | 4 (1) | 3 (0.3) | 4 (0.6) | 3 (0.3) | |
| Moderna | 152 (6.2) | 61 (9) | 68 (10.3) | 23 (2) | 95 (7.2) | 57 (5) | 56 (2.3) | 30 (4.4) | 21 (3.2) | 5 (0.4) | 31 (2.3) | 25 (2.2) | |
| ≤19 yr | 7 (21.6) | 2 (13.7) | 4 (31.5) | 1 (19.2) | 6 (37.6) | 1 (6.1) | 2 (6.2) | 1 (6.9) | 1 (7.9) | 0 (0) | 2 (12.5) | 0 (0) | |
| 20–29 yr | 61 (17.5) | 17 (11.6) | 42 (31.2) | 2 (2.9) | 46 (24.8) | 15 (9.2) | 16 (4.6) | 7 (4.8) | 8 (5.9) | 1 (1.5) | 10 (5.4) | 6 (3.7) | |
| 30–39 yr | 34 (8.8) | 17 (13.2) | 13 (10.3) | 4 (3.1) | 22 (9.8) | 12 (7.5) | 16 (4.2) | 11 (8.6) | 5 (4) | 0 (0) | 11 (4.9) | 5 (3.1) | |
| 40–49 yr | 20 (4.3) | 11 (6.4) | 7 (4.2) | 2 (1.6) | 8 (3.1) | 12 (5.8) | 11 (2.4) | 7 (4.1) | 3 (1.8) | 1 (0.8) | 3 (1.2) | 8 (3.9) | |
| 50–59 yr | 20 (3.3) | 13 (6.6) | 2 (1) | 5 (2.4) | 6 (1.9) | 14 (5) | 10 (1.6) | 4 (2) | 4 (2) | 2 (0.9) | 4 (1.2) | 6 (2.1) | |
| ≥60 yr | 10 (1.6) | 1 (5.2) | 0 (0) | 9 (1.5) | 7 (2.2) | 3 (1) | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.2) | 1 (0.3) | 0 (0) | |
| AstraZeneca | 12 (0.6) | 3 (0.3) | 9 (1) | 0 (0) | 8 (0.8) | 4 (0.4) | 9 (0.4) | 3 (0.3) | 6 (0.6) | 0 (0) | 6 (0.6) | 3 (0.3) | |
| ≤19 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 20–29 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 30–39 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 40–49 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 50–59 yr | 1 (0.4) | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 1 (0.8) | 1 (0.4) | 0 (0) | 1 (1.1) | 0 (0) | 1 (1.0) | 0 (0) | |
| ≥60 yr | 11 (0.7) | 2 (0.3) | 9 (1.1) | 0 (0) | 8 (1) | 3 (0.4) | 8 (0.5) | 3 (0.4) | 5 (0.6) | 0 (0) | 5 (0.7) | 3 (0.4) | |
| Janssen | 3 (2.0) | 3 (2.0) | 0 (0) | 0 (0) | 2 (1.6) | 1 (3.7) | 2 (1.3) | 2 (1.3) | 0 (0) | 0 (0) | 2 (1.6) | 0 (0) | |
| ≤19 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 20–29 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 30–39 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.0) | 1 (1.0) | 0 (0) | 0 (0) | 1 (1.2) | 0 (0) | |
| 40–49 yr | 3 (10.6) | 3 (10.9) | 0 (0) | 0 (0) | 2 (10.2) | 1 (11.6) | 1 (3.5) | 1 (3.6) | 0 (0) | 0 (0) | 1 (5.1) | 0 (0) | |
| 50–59 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| ≥60 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Novavax | 2 (3.7) | 2 (16.6) | 0 (0) | 0 (0) | 1 (4.0) | 1 (3.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| ≤19 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 20–29 yr | 1 (28.4) | 1 (62.5) | 0 (0) | 0 (0) | 0 (0) | 1 (53.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 30–39 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 40–49 yr | 1 (14.9) | 1 (37.0) | 0 (0) | 0 (0) | 1 (30.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 50–59 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| ≥60 yr | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
Unit: n (cases per million doses).
AstraZeneca, AstraZeneca COVID-19 vaccine (ChAdOx1-S); Janssen, Janssen COVID-19 vaccine (Ad26.COV2.S); Pfizer, Pfizer-BioNTech COVID-19 vaccine (BNT162b2); Moderna, Moderna COVID-19 vaccine (mRNA-1273); Novavax, Nuvaxovid prefilled syringe COVID-19 vaccine (NVX-CoV2373)





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


