Predictive factors of resistance to intravenous immunoglobulin and coronary artery lesions in Kawasaki disease
Article information
Abstract
Purpose
We conducted a study to determine which factors may be useful as predictive markers in identifying Kawasaki disease (KD) patients with a high risk of resistance to intravenous immunoglobulin (IVIG) and developing coronary artery lesions (CAL).
Methods
We enrolled 287 patients in acute phase of KD at a single center. The demographic, clinical and laboratory data were collected retrospectively.
Results
There were 34 patients in the IVIG resistant group. The IVIG resistant group had significantly higher serum N-terminal-pro-brain natriuretic protein (NT-proBNP) levels (P<0.01) and polymorphonuclear neutrophil (PMN) percentage (P<0.01) in comparison to the IVIG responders. The results yielded sensitivity (78.8%, 60.6%), specificity (58.2%, 90%) and cutoff value (628.6 pg/mL, 80.3%) of NT-proBNP and PMN respectively, in predicting IVIG resistance. Despite IVIG administration, 13 of the 287 patients developed CAL. The patients in the CAL group had higher NT-proBNP levels (P<0.01) and higher PMN percentage (P<0.01). In these patients, the results yielded sensitivity (73.3%, 56.7%), specificity (67.9%, 88.9%) and cutoff value (853.4 pg/mL, 80.3%) of NT-proBNP and PMN respectively, for predicting CAL. The area under the curve (AUC) for predicting resistance to IVIG was NT-proBNP 0.712, PMN 0.802. The AUC for predicting CAL was NT-proBNP 0.739, and PMN 0.773.
Conclusion
Serum NT-proBNP levels and PMN percentage were significantly elevated in patients with KD with IVIG resistance and CAL. Thus, they may be useful predicting markers for IVIG resistance and development of CAL in KD patients.
Introduction
Kawasaki disease (KD) is an acute febrile illness of childhood, a vasculitis of unknown etiology1), and is the most common cause of acquired cardiac disorders in pediatric patients2). There are no specific diagnostic laboratory markers for KD3).
Coronary artery complications develop in 20% to 25% of untreated KD patients4). Early detection and treatment reduce the incidence of serious coronary artery complications.
Recent studies have investigated factors for predicting resistance to intravenous immunoglobulin (IVIG) as well as coronary artery lesions (CALs) in patients with KD, but the results have been conflicting.
The purpose of our study was to determine which factors can be useful as predictive markers for patients with KD who are at higher risk of IVIG resistance and developing CAL.
Materials and methods
KD was diagnosed according to the criteria published by the American Heart Association (AHA) in 2004. Diagnosis of incomplete KD was based on the diagnostic criteria established by AHA2).
All patients received 50 mg/kg/day acetylsalicylic acid until they were afebrile and 2 g/kg of IVIG over 12 hours. The onset of KD including incomplete (atypical) KD was defined as the day of fever onset.
We conducted a retrospective review of medical records for 287 KD patients, admitted to Inje University Haeundae Paik Hospital between September 2010 and December 2015.
IVIG resistance was defined as persistent or recrudescent fever (temperature>38.0℃) after more than 36 hours following completion of IVIG infusion (2 g/kg). CALs were assessed by performing serial echocardiography, primarily by quantifying the internal coronary artery dimension as per the Japanese Ministry of Health criteria: a maximum absolute internal diameter >3 mm in children <5 years of age, or >4 mm in children 5 years and older, or segment 1.5 times greater than an adjacent segment, or the presence of luminal irregularity. We also diagnosed the presence of CAL whenever body surface area-adjusted Z score of any coronary artery was ≥+2.5, including left main, left anterior descending, and right coronary arteries2).
All laboratory data used in this study were obtained at admission before the initiation of IVIG therapy. The laboratory investigations included white blood cell count, polymorphonuclear neutrophil (PMN) percentage, albumin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), C-reactive protein (CRP), N-terminal-pro-brain natriuretic protein (NT-proBNP).
Statistical analysis was performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). A univariate analysis was performed on the clinical data of the KD patients in the IVIG resistant and CAL groups, in which categorical data were presented as as either significantly different or correlated. To assess the ability of laboratory factors for percentages and were analyzed using the Student t test. Quantitative data with normal distribution were presented as mean±standard deviation. A P<0.01 was considered predicting IVIG resistance and CAL development, the diagnostic performances of the parameters considered were investigated by receiver operating characteristic (ROC) analysis. This technique summarized the validity coefficients of a parameter and an overall index of diagnostic accuracy, the area under the curve (AUC) from a plot. Based on ROC analysis, the best statistical cutoff value for each parameter (the point at which the sum of false-positives and false-negatives is less than any other point) was calculated.
Results
Two hundred eighty-seven children (159 male and 128 female), age range: 2 months to 13 years 3 months (median age, 2.2 years), presenting with acute phase of KD were enrolled in this study. Patients with typical KD and incomplete KD accounted for 88.2% (n=253) and 11.8% (n=34), respectively.
1. Demographic, laboratory characteristics of patients at the diagnosis in the IVIG responsive group and the IVIG resistant group
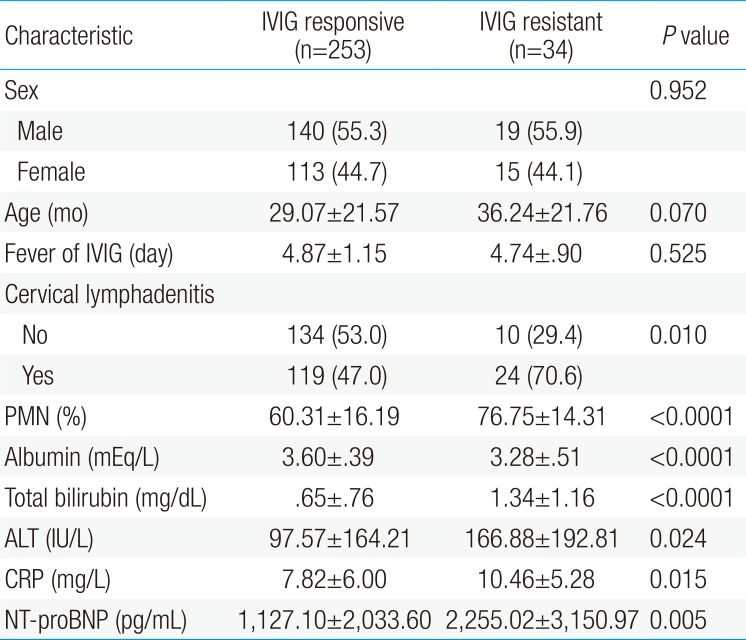
Out of the 287 patients, 253 (88.2%) responded to IVIG treatment, whereas 34 (11.8%) did not respond to the therapy. There were no significant differences between the IVIG resistant and IVIG responding groups based on gender (male/female, 19/15 and 140/113, respectively) and age (36.24±21.76 and 29.07±21.57 months, P=0.07). No significant differences were found for fever duration before IVIG between the IVIG resistant and IVIG responding groups (4.74±0.9 and 4.87±1.15 days, P=0.525).
The number of patients who developed CAL was significantly higher in the IVIG resistant group, 65% (21 of 34) in IVIG resistant group vs. 0.04% (9 of 253) in IVIG responding group (P<0.001). The laboratory data showed significant differences between the IVIG responding group and the IVIG resistant group (Table 1). Compared to the IVIG responding group, the IVIG resistant group had higher NT-proBNP levels (2,255.02±3,150.97 pg/mL vs. 1,127.10±2,033.60 pg/mL, P<0.01), higher serum CRP levels (10.46±5.28 mg/dL vs. 7.82±6.00 mg/dL, P=0.015), higher PMN percentage (76.7%±14.3% vs. 60.3%±16.2%, P<0.01), higher serum total bilirubin levels (1.34±1.16 mg/dL vs. 0.65±0.76 mg/dL, P<0.01), higher ALT levels (166.88±192.81 mg/dL vs. 97.57±164.21 mg/dL, P=0.024) and lower serum albumin levels (IVIG resistant vs. IVIG responding group: 3.28±0.51 mEq/L vs. 3.60±0.39 mEq/L, P<0.01).

Comparison of demographic and clinical characteristics between IVIG responsive and the IVIG resistant groups
The NT-proBNP cutoff value of 628.6 pg/mL yielded a sensitivity of 78.8%, a specificity of 58.2% for predicting IVIG resistant group in the acute phase of KD. The CRP cutoff value of 6.81 mg/dL yielded a sensitivity of 81.8% and a specificity of 54.2% for predicting IVIG resistance. The PMN cutoff value of 80.3% yielded a sensitivity of 60.6% and a specificity of 90% for predicting IVIG resistance.
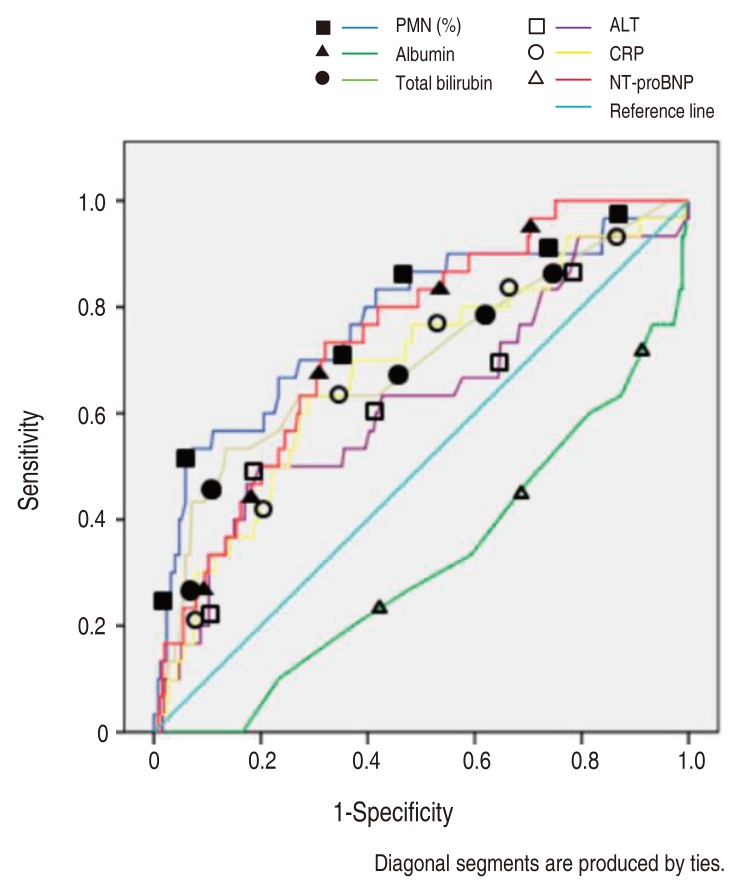
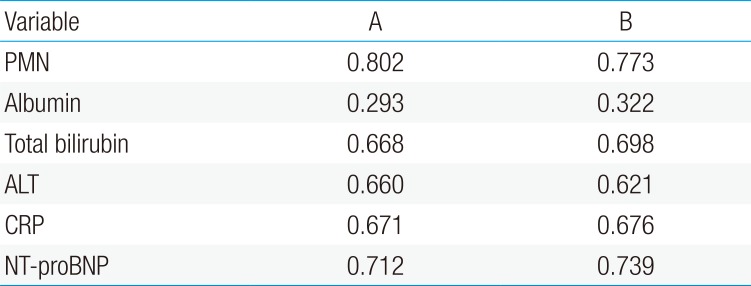
The NT-proBNP levels significant for predicting IVIG resistance in KD patients were assessed by a ROC curves analysis (Fig. 1). The AUC for predicting resistance to IVIG (Table 2) with various variables were: PMN 0.802, serum NT-proBNP 0.712, CRP 0.671, total bilirubin 0.668, ALT 0.66, AST 0.637 and serum albumin 0.293.
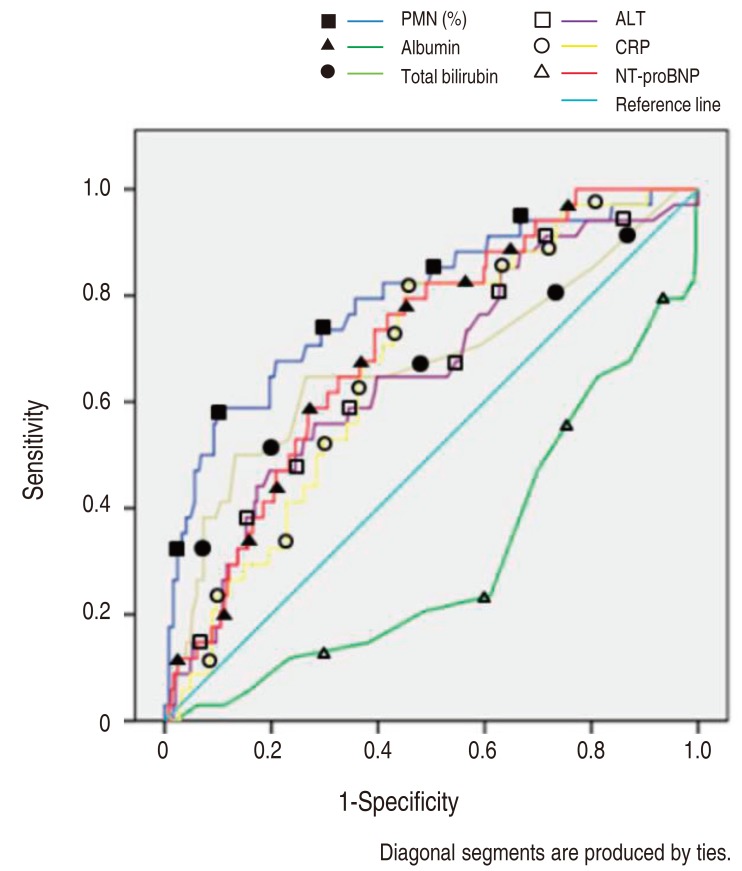
Receiver-operating characteristic curves comparing the sensitivity and specificity of various variables (resistance to intravenous immunoglobulin treatment). PMN, polymorphonuclear neutrophil percentage; ALT, alanine aminotransferase; CRP, C-reactive protein; NT-proBNP, N-terminal-pro-brain natriuretic protein.
2. Demographic, laboratory characteristics of patients at the diagnosis in the group without CAL and with CAL
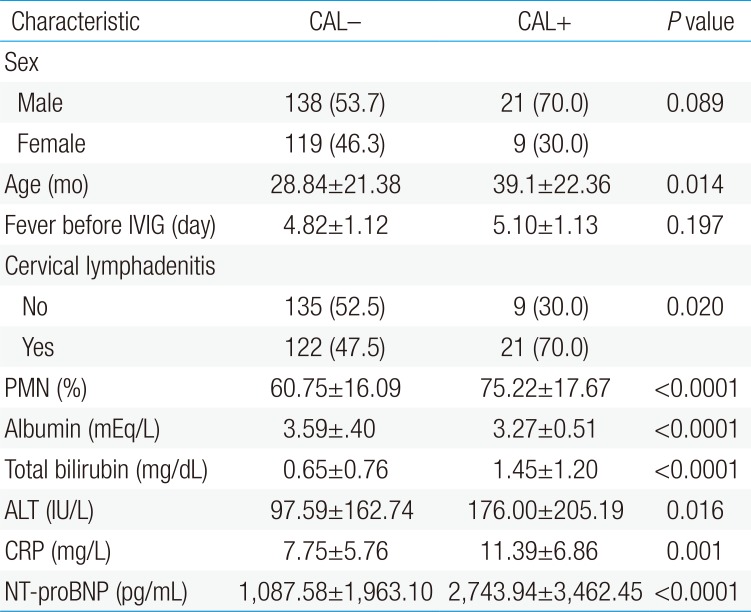
Thirty patients from the total study population of 287 patients developed CAL, despite receiving IVIG. There were significant differences in age between the CAL and non-CAL groups (39.1±22.36 and 28.84±21.38 months, P=0.014). No significant difference was found for fever duration before IVIG (5.10±1.13 and 4.82±1.12 days). The patients in the CAL group had higher NT-proBNP levels (2,743.94±3,462.45 pg/mL vs. 1,087.58±1,963.10 pg/mL, P<0.01), higher serum CRP levels (11.39±6.86 mg/dL vs. 7.75±5.76 mg/dL, P<0.01), a higher PMN percentage (75.2%±17.6% vs. 60.7%±16.0%, P<0.01), higher serum total bilirubin levels (1.45±1.20 mg/dL vs. 0.65±0.76 mg/dL, P<0.01), higher ALT levels (176±205.19 mg/dL vs. 97.59±162.74 mg/dL, P=0.016) and lower serum albumin levels (3.27±0.51 mEq/L vs. 3.59±0.40 mEq/L, P<0.01), in comparison with the patients in the non-CAL group (Table 3).
An NT-proBNP cutoff value of 853.4 pg/mL yielded a sensitivity of 73.3%, a specificity of 67.9% for predicting CAL in the acute phase of KD. The CRP cutoff value of 9.4 mg/dL yielded a sensitivity of 63.3% and a specificity of 71.6% for predicting CAL. The PMN percentage cutoff value of 80.3% yielded a sensitivity of 56.7% and a specificity of 88.9% for predicting CAL.
The NT-proBNP level for predicting CAL in patients with KD was assessed by an ROC curves analysis (Fig. 2). The AUC for predicting CAL with various variables were as follows: PMN percentage, 0.773; serum NT-proBNP, 0.739; CRP, 0.676; total bilirubin, 0.698; ALT, 0.621; serum albumin, 0.322 (Table 2).
Discussion
The diagnostic criteria of KD were set forth over 40 years ago. Despite several revisions over the years, they are still insufficient for adequate diagnosis of the disease. Unfortunately, there are no diagnostic tests currently available that help early diagnosis of KD patients. Our results suggest that the serum NT-proBNP levels and PMN percentage are significantly elevated in patients with KD with IVIG resistance and CAL and may be useful predicting markers.
Some patients with KD fail initial IVIG therapy for reasons that are not clear. Retrospective studies have identified potential factors that could predict which patients will require further therapy for refractory disease. The presence of one or more of these risk factors for treatment failure should alert clinicians to an increased likelihood that the patient may not respond adequately to the initial IVIG therapy. The ability to predict a lack of response to IVIG before initiating therapy, would allow clinicians to identify these patients because they might benefit from more aggressive treatment.
Several previous studies reported increased patient serum NT-proBNP levels in acute phase of KD, thus providing useful markers for diagnosing KD5678910). In addition, several studies have published data that NT-proBNP could be used for predicting resistance to IVIG therapy and patients who are at high risk for CAL111213).
The NT-proBNP is a prohormone with a 76 amino acid N-terminal inactive protein that is cleaved from the molecule to release brain natriuretic peptide (BNP). BNP is a diuretic peptide that is secreted mainly from cardiac ventricles. BNP and NT-proBNP has been correlated with echocardiographic measurements as a suggestive marker for diastolic dysfunction1415).
The mechanisms by which the elevated levels of serum NT-proBNP occur in the acute phase of KD are not clear at the present time, however there is a possibility that proinflammatory cytokines such as tumor necrosis factor-α or interleukin-1 beta, known to be increased in the acute phase of KD, induce the secretion of BNP from myocytes16). A linear correlation between NT-proBNP and CRP levels was present in the current study ([NT-proBNP= 0.147 CRP+103.936]; P<0.01) (Fig. 3).
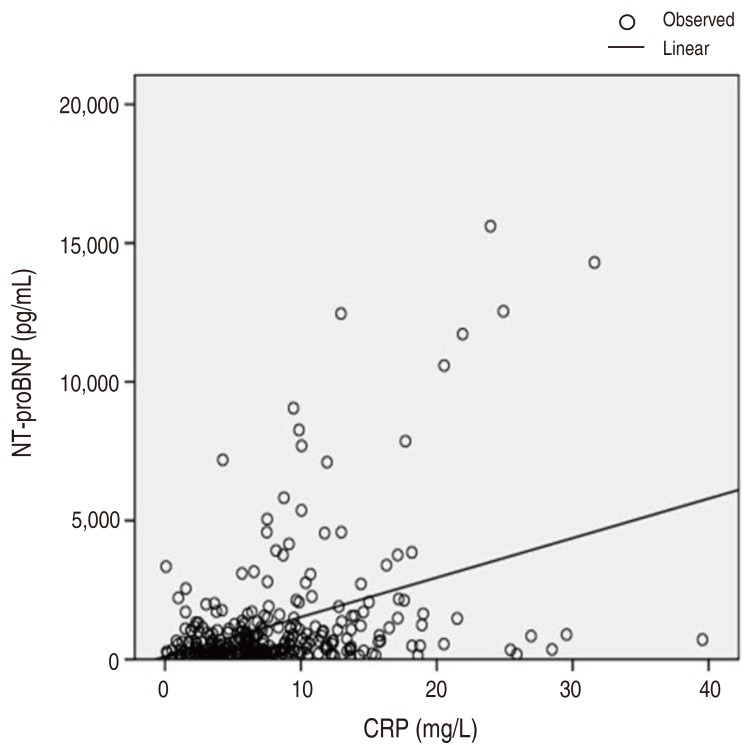
Correlation between elevated N-terminal-pro-brain natriuretic protein (NT-proBNP) levels and C-reactive protein (CRP).
The serum concentration of NT-proBNP may be useful for predicting IVIG resistance and CAL in patients with the acute phase of KD. It is worthwhile to consider and evaluate risk stratified strategies for patients at a higher risk of developing severe KD, based on the serum concentration of NT-proBNP.
Kaneko K et al.11) previously reported that NT-proBNP can be a useful biomarker to predict which patients with KD are at greater risk of CAL before initial IVIG. And in another study by Yoshimura et al.12), they further confirmed that the serum NT-proBNP levels increased in KD children with CAL in a larger sample and also found that it was increased in IVIG nonresponders. In that study, 19 of the 80 patients developed CAL, despite IVIG administration. They had significantly higher serum NT-proBNP levels in comparison with the patients without CAL. The NT-proBNP cutoff value of 1,300 pg/mL yielded a sensitivity of 95% and a specificity of 85% for predicting CAL. However, 17 of the 80 patients were IVIG nonresponders. They also had significantly higher serum NT-proBNP levels in comparison with the IVIG responders. The NT-proBNP cutoff value of 800 pg/mL yielded a sensitivity of 71% and a specificity of 62% for predicting IVIG nonresponders. Kim et al.13) also showed that NT-proBNP is a helpful marker in determining patients at risk for not responding to initial IVIG treatment.
By using larger data in our study, we further confirmed that the serum NT-proBNP levels are also increased in children with KD, having CAL and IVIG resistance.
By the AUC, the serum levels of PMN percentage, NT-proBNP, and CRP were considered to be good predictors for IVIG resistance in KD patients. The serum levels of NT-proBNP and PMN percentage were considered to be good predictors for developing CAL in KD patients. These results were compared to previous study. In that study, the serum level of NT-proBNP and serum CRP were considered to be better single predictor for IVIG nonresponders. And the serum level of NT-proBNP was considered to be the best single predictor for CAL among these biochemical markers in patients with KD12).
Moreover, our study also revealed significantly higher levels of total bilirubin and ALT. However serum albumin levels were significantly lower in both IVIG resistant and CAL groups (Tables 1, 3). These values correlated with the results of our previous meta-analysis study of laboratory predictive factors for IVIG resistant KD. Meta-analysis of twelve studies, comprising 2,745 patients, demonstrated that laboratory predictive factors for IVIG resistant KD included higher levels of total bilirubin, PMN percentage, NT-proBNP, ALT, CRP, and lower sodium and albumin17).
In our study, PMN percentage was an important laboratory predictive factor in addition to NT-proBNP. Our results correlated with the findings of Ha et al.18). In their study in patients with acute febrile phase of KD, IVIG resistant patients had higher neutrophil-to-lymphocyte ratios (NLR) than IVIG responsive patients. They also observed higher NLR in patients with aneurysms, but not in patients with dilatation, compared to that in patients without coronary artery abnormalities. Their study concluded that an NLR>1 two days after IVIG was predictive of coronary aneurysm development and IVIG resistance.
While controversy remains as to which risk factors predict patient resistance to IVIG, and treatment regimens to reduce the risk of developing CAL, the presence of several predictive factors in our study, especially proBNP and PMN percentage, should alert clinicians to the increased probability of the patient not responding adequately to initial IVIG therapy and developing CAL.
In conclusion, the serum NT-proBNP, PMN percentages, CRP and total bilirubin are increased significantly in KD patients with IVIG resistance and CAL. Serum NT-proBNP levels and PMN percentage may be useful for predicting IVIG resistance and CAL in KD.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.