Endocrine comorbidities of pediatric obesity
Article information
Abstract
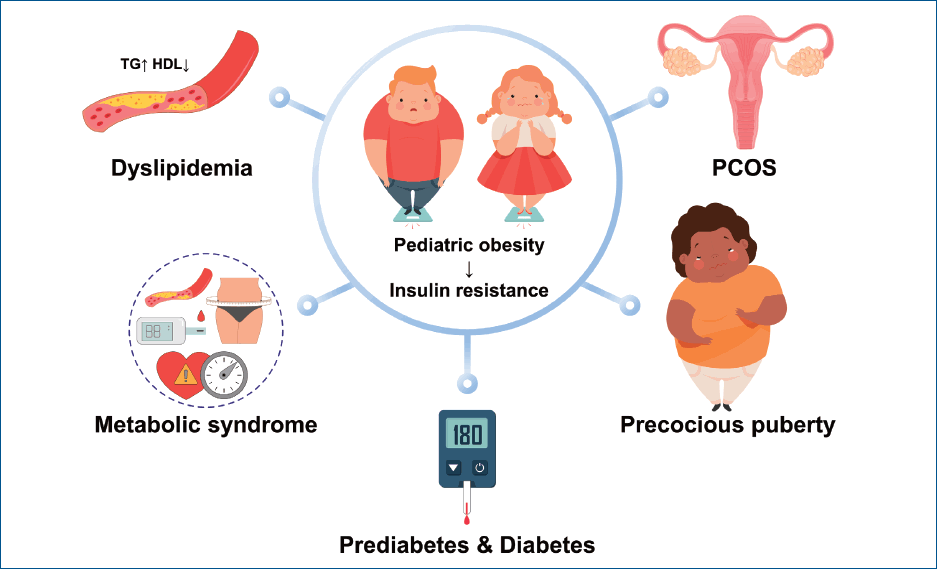
Pediatric obesity has become a serious public health issue. The prevalence of obesity in children and adolescents has increased worldwide and in Korea over several decades. Obese children are more likely to be obese adults with an increased cardiovascular risk. Therefore, maintaining a healthy weight and preventing obesity during childhood are of critical importance. Moreover, obese children and adolescents often have endocrine comorbidities such as prediabetes, type 2 diabetes, dyslipidemia, metabolic syndrome, polycystic ovary syndrome, and central precocious puberty. Hence, the early implementation of obesity management using a multidisciplinary team approach and screening for these comorbidities in obese children and adolescents are required with the appropriate management of each comorbidity and/or specialist referral.
Key message
· Pediatric obesity can involve endocrine comorbidities such as prediabetes, type 2 diabetes, dyslipidemia, metabolic syndrome, polycystic ovary syndrome, and central precocious puberty.
· Prediabetes and type 2 diabetes in youth aged 10-19 years had a prevalence of 25.9% and 0.6% in 2013-2014, respectively.
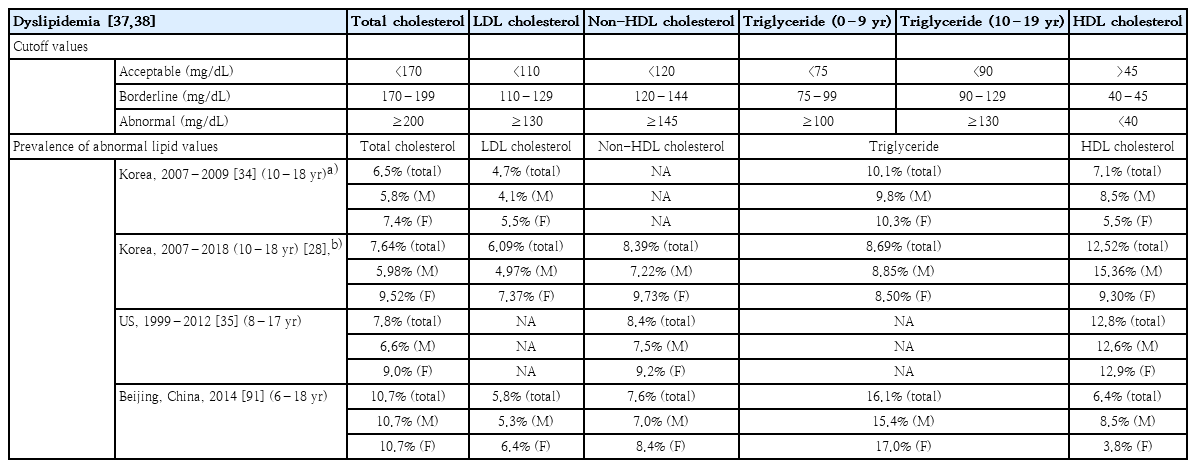
· Dyslipidemia in Korean adolescents aged 10-18 years had a prevalence of 7.64% (total cholesterol ≥200 mg/dL), 6.09% (low-density lipoprotein cholesterol ≥130 mg/dL), 8.69% (triglyceride ≥150 mg/dL), and 12.52% (high-density lipoprotein cholesterol ≤40 mg/dL) in 2007-2018.
· Metabolic syndrome in Korean youth has a prevalence of 1.9%-14.7% in males and 1.7%-12.6% in females with wide variation in definitions.
· Appropriate comorbidity screening and management and/or specialist referral are necessary for obese children and adolescents.
Graphical abstract
Introduction
Obesity is a state of excessive fat accumulation [1]. Body mass index (BMI), calculated as weight in kilograms divided by height in meters squared, is commonly used to diagnose obesity. BMI is closely correlated with body fat, is easily calculated, and has reference values for comparison. In children aged 2–20 years, obesity is defined as a BMI ≥95th percentile and overweight as a BMI ≥85th percentile but <95th percentile of the corresponding sex and age [2]. In children aged <2 years, obesity is defined as a sex-specific weight for a length ≥97.7th percentile [2].
The prevalence of pediatric obesity has increased worldwide. Between 1975 and 2016, its worldwide prevalence in children and adolescents aged 2–18 years increased from 0.7% to 5.6% in girls and from 0.9% to 7.8% in boys [3]. In Korea, the prevalence of obesity in the pediatric population aged 2–18 years increased from 5.8% in 1997 to 9.7% in 2005 [4]. Among school-aged children aged 6–18 years, the prevalence of obesity progressively increased from 8.7% in 2007 to 15.0% in 2017 [5]. Extreme obesity has increased significantly among children and adolescents aged 10–19 years, especially among boys [6].
Pediatric obesity has become a serious public health issue. Obese children are more likely to be obese adults. In a meta-analysis, the pooled relative risk for adult obesity among obese children is more than 5 times higher than that among their nonobese counterparts [7]. Moreover, pediatric obesity increases one’s cardiovascular risk in adulthood [8]. Recent epidemiological studies demonstrated an association between childhood obesity and an increased risk of several cancers in adulthood, such as leukemia, Hodgkin’s disease, colorectal cancer, and breast cancer [9,10]. The lifetime costs for those with a history of childhood obesity are 3–5 times higher than those of their nonobese counterparts [11,12]. Pediatric obesity is accompanied by many comorbidities, including those in the endocrine, pulmonary, cardiovascular, gastrointestinal, renal, and/or other systems [2].
This review article discusses the endocrine comorbidities of pediatric obesity, such as prediabetes, type 2 diabetes (T2D), dyslipidemia, metabolic syndrome (MS), polycystic ovary syndrome (PCOS), and central precocious puberty (CPP).
Prediabetes and T2D
Diabetes mellitus is a disease characterized by chronic hyperglycemia caused by abnormal insulin secretion or resistance that results in metabolic disturbances [13]. Prediabetes is a state with high blood glucose levels between normoglycemia and diabetes that indicates an increased risk of developing diabetes, including impaired fasting glucose, impaired glucose tolerance, elevated glycated hemoglobin (HbA1c), or a combination thereof [14]. Both prediabetes and diabetes are associated with cardiovascular diseases (CVDs) in adulthood.
Pediatric obesity is associated with an increased risk of prediabetes and diabetes, especially T2D [15]. Obesity increases insulin resistance in the skeletal muscles, liver, and adipose tissues, leading to prediabetes and T2D [13]. Excessive ectopic fat accumulation in the skeletal muscles and liver exacerbates peripheral insulin resistance [16]. Insulin resistance causes decreased glucose disposal in the skeletal muscles, increased glucose production in the liver, and the increased production of free fatty acids (FFAs) and inflammatory cytokines in the adipose tissues [17]. Beta cell function and mass are also gradually decreased due to glucotoxicity, lipotoxicity, mitochondrial dysfunction, and endoplasmic reticular stress [18]. T2D in children and adolescents differs from adult-onset T2D, including the early deterioration of beta cell function and rapid development of diabetic complications [19,20].
Diabetes and prediabetes are diagnosed according to American Diabetes Association (ADA) guidelines [14]. Prediabetes is defined as a fasting plasma glucose ≥100 mg/dL but <126 mg/dL, a 2-hr glucose level after an oral glucose tolerance test ≥140 mg/dL and <200 mg/dL, or a HbA1c level ≥ 5.7% but <6.5%. Diabetes is defined as a fasting plasma glucose ≥ 126 mg/dL, a 2-hr glucose level after an oral glucose tolerance test ≥ 200 mg/dL, an HbA1c ≥ 6.5%, or a random glucose level ≥ 200 mg/dL with classical symptoms including fatigue, polyuria, and weight loss. The usefulness of HbA1c for the diagnosis of prediabetes and T2D in pediatric patients has yet to be determined [14,21].
ADA and the International Society for Pediatric and Adolescent Diabetes recommend risk-based screening strategies for prediabetes and T2D in asymptomatic youth [14,22]. Overweight or obese youth with 1 or more of the following risk factors, who are in puberty or aged ≥10 years, are recommended to undergo screening: maternal diabetes or gestational diabetes; family history of diabetes; signs of or conditions related to insulin resistance, including acanthosis nigricans, hypertension, dyslipidemia, PCOS, or born small for gestational age; or race/ethnicity [14,22]. Screening tests include fasting plasma glucose, 2-hour plasma glucose after an oral glucose tolerance test, and HbA1c [23]. Follow-up tests are recommended at least at 3-year intervals when the test result is normal. However, more frequent tests are required if the individual’s BMI increases or other risk factors worsen [14,22].
Recent epidemiologic data revealed an increasing prevalence of pediatric prediabetes and T2D along with an increase in obesity (Table 1) [13]. In the United States (US), among pediatric diabetes cases, the prevalence of T2D in children and adolescents increased from approximately 3% in the early 1990s to 20% in 2003 [24,25]; the prevalence of prediabetes in youth aged 12–18 years was 18.0% in 2005–2016 [26]. In Korea, the estimated prevalence of prediabetes and T2D in youth aged 10–19 years according to nationally representative data showed an increasing tendency from 0.6% in 2005 to 25.9% in 2013–2014 for prediabetes and from 0.1% in 2007 to 0.6% in 2013–2014 for T2D [27]. In China, the prevalence of impaired fasting glycemia and T2D showed more than a 2-fold increase between 2002 and 2012 [28].
Consensus is lacking on the treatment of children and adolescents with prediabetes. However, lifestyle modifications are generally recommended, including a balanced and healthy diet, increased physical activity, and decreased sedentary behavior [29]. In pediatric patients with T2D, lifestyle management and diabetes education should be initiated at the time of diagnosis[22,30]. Metformin and insulin, alone or in combination, are used for pharmacologic treatment of T2D [22]. The choice of initial pharmacologic treatment depends on the severity of hyperglycemia and metabolic disturbances such as acidosis or ketosis [22].
The glycemic target for youth with T2D is an HbA1c level <7%, although the level should be individualized according to a patient’s circumstances [31]. If glycemic targets are not met, increments in the dose of metformin and the addition or titration of insulin dose should be implemented. If HbA1c targets are not met with metformin and insulin, liraglutide might be considered [32]. However, as of early 2020, this drug was not yet approved for adolescents in Korea. Metabolic bariatric surgery is selectively and limitedly considered in cases of uncontrolled T2D with severe obesity and serious comorbidities [33].
To prevent diabetic complications, the measurement of blood pressure at each visit and annual urinary albumin-to-creatinine ratio, fasting lipid panel, and retinal examinations should be performed after the diagnosis of T2D is made [34].
Dyslipidemia
Dyslipidemia, abnormal blood lipid levels caused by disordered lipoprotein metabolism [35], is a risk factor for CVD and among the main causes of mortality and morbidity in adults [35]. Since dyslipidemia and atherosclerotic vascular changes also begin in childhood and adolescence [36], the early detection and proper management of dyslipidemia could prevent atherosclerosis and CVD in adulthood.
Dyslipidemia includes elevated total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglyceride, non-high-density lipoprotein cholesterol (non-HDL-C) levels, and a decreased HDL-C level. Lipid levels in children and adolescents are classified as acceptable, borderline, or abnormal (Table 2). The cutoff values were derived from population-based studies and are used in several guidelines [37,38].
Obesity is a risk factor for dyslipidemia [39]. Lipid abnormalities commonly observed in obese populations are elevated triglycerides, very LDL (VLDL), apolipoprotein B, and non-HDL-C and decreased HDL-C [39]. Insulin resistance, visceral adiposity, and a proinflammatory state in obesity are associated with increased basal lipolysis in the adipose tissues, which leads to elevated circulating FFA levels [39]. An increased influx of FFAs to the liver contributes to the hepatic overproduction of VLDL particles. Serum triglyceride levels increase due to increases in VLDL particles and the decreased clearance of triglyceride-containing lipoproteins [39]. Increased triglyceride levels are associated with decreased HDL-C levels via cholesteryl ester transfer protein-mediated reciprocal exchange [39].
The prevalence of dyslipidemia in Korean adolescents aged 10–18 years was 6.5% for total cholesterol ≥200 mg/dL, 4.7% for LDL-C ≥130 mg/dL, 10.1% for triglyceride ≥150 mg/dL, and 7.1% for HDL-C <35 mg/dL in 2007–2009 [40]. The prevalence of dyslipidemia in US children and adolescents aged 8–17 years decreased between 1999–2000 and 2011–2012: 10.6% to 7.8% for total cholesterol ≥200 mg/dL and 13.6% to 8.4% for non-HDL-C ≥145 mg/dL [41]. However, the prevalence of dyslipidemia increased with the degree of obesity in US children and young adults [8].
Several guidelines for dyslipidemia in children and adolescents recommend “universal screening” at the ages of 9–11 and 17–21 years using nonfasting non-HDL-C testing [37,38,42]. However, since obesity is a risk factor for dyslipidemia, lipid screening in obese children and adolescents is required at age ≥ 2 years. If nonfasting non-HDL-C is ≥145 mg/dL, a fasting lipid panel should be performed twice within 3 months [37,38]. The average values are used for management decisions.
The management of dyslipidemia in children and adolescents begins with lifestyle changes including diet and exercise [37]. The Cardiovascular Health Integrated Lifestyle Diet (CHILD-1) is recommended for children aged ≥2 years. If the fasting lipid profile remains abnormal for 6 months, the CHILD-2-LDL or CHILD-2-TG diet should be applied according to the lipid abnormalities [37]. Along with dietary management, moderate to vigorous physical activity for at least 1 hour daily and decreased sedentary time are essential [37].
Pharmacologic treatment for children and adolescents with dyslipidemia is generally recommended for those aged ≥10 years who show a poor response to lifestyle modifications. Treatment with statins is recommended for those with an elevated LDL-C (≥190 mg/dL); an LDL-C of 160–189 mg/dL with a family history, ≥1 high-risk factor, or ≥2 moderate-risk factors; or an LDL-C of 130–159 mg/dL with ≥2 high-risk factors, 1 high-risk factor and ≥2 moderate-risk factors, or clinical CVD [37]. For those with elevated triglyceride levels, omega-3 fish oil or fibric acid derivatives must be considered [37]. The treatment target for LDL-C is <130 mg/dL. Fasting lipid profiles and the evaluation of adverse effects must be performed regularly [37].
Metabolic syndrome
MS is defined as a constellation of several cardiometabolic risk factors. The concept of MS was first described by Raven in 1988 as a cluster of cardiometabolic risk factors associated with insulin resistance; he named it “syndrome X.” [43] In 2001, the term “metabolic syndrome” was first used by the National Cholesterol Education Program–Adult Treatment Panel III (NCEP–ATP III), with the presence of 3 of 5 components including abdominal obesity, hypertension, hyperglycemia, hypertriglyceridemia, and a low HDL-C level [44]. Since then, several definitions for the diagnosis of MS have emerged for adult and pediatric populations.
In the pediatric population, the same components as adult populations, namely waist circumference, blood pressure, fasting glucose, fasting triglyceride, and fasting HDL-C, are used to diagnose MS. However, consensus is lacking about a single unified definition of pediatric MS. The difficulties developing diagnostic criteria for pediatric MS stem from the rapid growth and hormonal changes during puberty, physiologic insulin resistance and its effect on lipid metabolism, and gender differences in fat mass and distribution [45]. Among the many diagnostic criteria suggested for pediatric MS, the modified NCEP–ATP III criteria by Cook et al. [46], International Diabetes Federation (IDF) criteria [47], and the criteria by de Ferranti et al. [48] are widely used (Table 3).
The reported prevalence of MS in children and adolescents varies widely among definitions and study populations [49]. In Korean youth, the prevalence of MS was as follows: 13.7% (males, 14.7%; females, 12.6%) among those aged 12–19 years in 1999–2012 according to the criteria by de Ferranti et al. criteria [50]; 5.7% (males, 5.8%; females, 5.5%) among those aged 10–18 years in 2010–2012 according to the modified NCEP–ATP III criteria [51]; and 1.8% (males, 1.9%; females, 1.7%) among those aged 10–19 years in 2011–2014 according to the IDF criteria [52]. Among US youth, the prevalence of MS was 4.2% (males, 6.1%; females, 2.1%) among those aged 12–19 years in 1988–1994 according to the modified NCEP–ATP III criteria [46]; 12.9% for Mexican Americans, 10.9% for non-Hispanic whites, and 2.5% for non-Hispanic Blacks aged 12–19 years in 1988–1994 according to the criteria by de Ferranti et al. [48]; and 4.5% (males, 6.7%; females, 2.1%) among those aged 12–17 years in 1999–2004 according to the IDF criteria [53].
Although the pathogenesis of MS is not fully understood, obesity, insulin resistance, inflammation, and their interplay are considered cornerstones of MS that affect the development and progression of each of its components [54]. Moreover, genetic and environmental risk factors are known to contribute to the development of MS, such as family history, female sex, decreased physical activity, increased sedentary time, and smoking [55].
The long-term consequences of MS in childhood are T2D, CVD, and other obesity-related disorders in adulthood. Youth with MS have a 2–3 times higher risk of developing T2D in adulthood than those without MS [56]. MS in childhood showed an increased odds ratio of 14.6 for adult CVD in a 25-year follow-up study [57]. Moreover, MS in childhood is more likely to track into adulthood [56,57].
The treatment of MS in children and adolescents consists of lifestyle modifications and specific treatment for each component of MS [58]. Lifestyle modification includes weight reduction with a healthy diet, increased physical activity, and decreased sedentary time. At least 20 minutes of daily moderate to vigorous physical activity is recommended [2]. Engagement of the entire family is required to ensure effective lifestyle changes. Hypertension should first be managed with lifestyle interventions such as reduced salt intake, weight reduction, and increased physical activity. If hypertension persists after 6 months of lifestyle changes, pharmacological treatment should be considered [59]. In cases of MS, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers are recommended for the treatment of hypertension [59,60]. Treatments for prediabetes, T2D, and dyslipidemia are described in the sections above.
Polycystic ovary syndrome
PCOS is characterized by otherwise unexplained hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphologic features [60]. PCOS is among the most common endocrine disorders of women of reproductive age, with a prevalence of 5%–10% [61]. The prevalence of PCOS in adolescents differs among criteria, ranging from 3.4% to 11.0% [62]; however, there are no epidemiological data on PCOS in Korean adolescents. PCOS in adolescents is associated with decreased quality of life [63], mental health problems [64,65], metabolic disturbances, and long-term poor cardiovascular and reproductive outcomes [66].
Although the pathophysiology of PCOS remains to be determined, hyperandrogenism, insulin resistance, and imbalanced gonadotropin secretion are key contributors [60]. PCOS is commonly observed in overweight or obese women, and the increasing prevalence of obesity may substantially influence the development of PCOS in susceptible individuals [60]. Nonetheless, obesity and other adipose tissue-related factors can aggravate pre-existing clinical, hormonal, and metabolic features in women with PCOS and may play a crucial role in the progression and/or maintenance of PCOS [60]. Recent studies revealed that genetic and environmental factors are associated with the development of PCOS [67].
The diagnosis of PCOS in adolescents is based on clinical and biochemical characteristics. However, consensus is lacking on the diagnostic criteria for PCOS among youth. The common presenting symptoms and signs of PCOS include menstrual irregularity, including oligomenorrhea and amenorrhea, signs of insulin resistance, such as acanthosis nigricans, and signs of hyperandrogenism, including hirsutism and acne [60]. Laboratory tests are performed to confirm hyperandrogenism, exclude other causes, and evaluate accompanying metabolic disturbances [68]. Pelvic ultrasonography might be helpful for excluding other causes of hyperandrogenism such as tumors. Making the diagnosis of PCOS in adolescents might be challenging because discriminating between physiological anovulation during puberty and ovulatory dysfunction in PCOS is demanding.
The treatment goals of PCOS are restoring a normal menstrual cycle, decreasing the signs of hyperandrogenism, improving quality of life, preserving fertility, and reducing the long-term risk of metabolic, cardiovascular, and reproductive outcomes [69,70]. The management of adolescents with PCOS should be individualized. Lifestyle modification is the first-line intervention for the treatment of PCOS, including weight reduction through a healthy diet and increased physical activity. Combined oral contraceptives (COCs) are the first-line pharmacologic treatment for PCOS to improve menstrual regularity and reduce androgen excesses. Anti-androgens could be used in conjunction by those who are unresponsive to COCs [67]. Metformin, an insulin-sensitizing agent, is recommended for patients with PCOS and dysglycemia [67]. Metformin improves metabolic disturbances and menstrual irregularities. Topical treatment of the local effects on hirsutism may be selectively applied [67]. More data on pharmacologic therapy for adolescents with PCOS are required.
Central precocious puberty
CPP, which is caused by premature activation of the hypothalamic-pituitary-gonadal (HPG) axis, results in the early manifestation of secondary sexual characteristics [71]. CPP is defined as breast development before 8 years of age in girls and testicular enlargement before 9 years of age in boys [72].
Obesity is associated with the early initiation of pubertal timing [73]. The recent increase in the prevalence of pediatric obesity might be associated with secular trends toward an earlier onset of puberty. Many epidemiologic studies revealed that pediatric obesity is associated with early puberty [74,75]. In Korea, the prevalence of CPP has been increasing with obesity [76], and the menarcheal age has been decreasing [77]. In obese children, insulin resistance with compensatory hyperinsulinemia and hyperandrogenism are the putative factors that initiate early HPG axis activation [78]. Adipokines, including leptin and adiponectin, which affect kisspeptin release, can also stimulate the initiation of puberty [79].
CPP is confirmed by a gonadotropin-releasing hormone (GnRH) stimulation test in children with early signs of puberty and greater bone age than chronological age [80]. The optimal cutoff value of peak GnRH stimulated luteinizing hormone level for identifying children with CPP is ≥5 IU/L. All boys with CPP and girls diagnosed with CPP before the age of 6 years should undergo brain imaging to screen for a central nervous system etiology [71].
The treatment of CPP halts the progression of the rapid development of pubertal signs, prevents the impairment of final adult height, and mitigates psychological issues [71]. The treatment of choice is the regular injection of the depot formulation of GnRH agonist [81]. In Korea, 1-, 3-, and 6-month depot formulations have been approved. The long-term use of GnRH agonists is generally safe [71].
Conclusion
With the increased prevalence of pediatric obesity, endocrine comorbidities also show increasing trends, including prediabetes and T2D, dyslipidemia, MS, PCOS, and CPP. These cardiometabolic risks in childhood track into adulthood. Therefore, maintaining a healthy weight and preventing obesity are of critical importance. In obese children and adolescents, the early implementation of obesity management through a multidisciplinary team approach and screening for comorbidities are required. In cases of comorbidities, appropriate management and/or specialist referrals are necessary.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.