Application of 3-dimensional printing implants for bone tumors
Article information
Abstract
Three-dimensional (3D) additive manufacturing has recently been used in various medical fields. Among them, orthopedic oncology is one that utilizes it most actively. Bone and tumor modeling for surgical planning, personalized surgical instrument fabrication, and implant fabrication are typical applications. The 3D-printed metal implants using titanium alloy powder have created a revolutionary change in bone reconstruction that can be customized to all body areas; however, bioprinting remains experimental and under active study. This review explores the practical applications of 3D printing in orthopedic oncology and presents a representative case. The 3D-printed implant can replace the conventional tumor prosthesis and auto/allobone graft, thereby personalizing bone reconstruction. Biologic bone reconstruction using biodegradable or bioprinted materials beyond metal may be possible in the future.
Key message
∙ The application of 3-dimensional (3D) printing in orthopedic oncology is summarized into bone and tumor modeling, patient-specific instruments (PSIs), custom-made implants, and tissue engineering.
∙ The 3D-printed customized implant is the most central application, while modeling and PSI often play adjunct roles.
∙ Short-term surgical outcomes of custom-made 3D-printed implants are promising.
Graphical abstract. Flow chart of the 3-dimensional (3D)-printing of the personalized surgical instrument and implant fabrication in orthopedic oncology.
Introduction
Additive manufacturing (AM), also called 3-dimensional (3D) printing, was conceptually attempted in 1981 by Hideo Kodama, and Charles Hull filed a patent application in the United States in 1986 [1-6]. There are many types of 3D printers that use various materials. Metals, polymers, hydrogels, and living cells are intended for 3D printing use in the medical field. Metals and polymers are currently being 3D-printed in the medical field. When living cells are printed as organs/organ-like objects, the process is called bioprinting.
AM has 2 advantages that enable its use in the medical field. First, AM enables the manufacturing of shapes that could not be made using traditional production methods [5,7,8]. Specifically, a stacking structure with different materials and a planned internal porosity can be created using AM. A lattice structure with a planned internal porosity has unique mechanical properties and provides a scaffold for tissue integration. Moreover, stacking with a hydrogel layer and a living cell layer is the key mechanism of bioprinting. Second, the cost of products fabricated by AM remains constant, even when mass production is difficult because of diversification [9,10]. To achieve personalized medicine, many medical products must be personalized for each patient, for which AM is suitable.
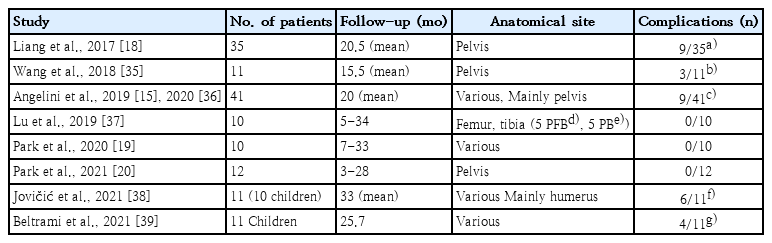
In the medical field, orthopedic oncology is one that utilizes 3D-printing most actively. Malignant bone tumors can affect any part of the skeleton to various extents with or without joint or growth plate involvement. Moreover, bone tumors, such as osteosarcoma, have a bimodal incidence and more often affect young patients with small bones than adults. In orthopedic oncology, complete bone-tumor excision with safe margins, which provides an appropriate normal tissue envelope around the tumor and enables the reconstruction of massive bone defects, are two of the main steps of limb salvage surgery for malignant bone tumors. Therefore, limb salvage surgery represents a wide variety of surgeries, and all are highly individualized. In orthopedic oncology, modular endoprosthesis with multiple pieces assembled during surgery is most commonly used to insert implants of various sizes. However, modular implants are not available in all circumstances. For example, custom-made implants are required for the reconstruction of unusual sites such as the pelvis or small bones in children. Another major indication for 3D-printing custom-made implants is in cases of anticipated improved surgical results. When a bone tumor is close to the joint or physis, limb salvage surgery using conventional implants is often performed as arthroplasty and sacrifices the joint. However, with a 3D-printed custom-made implant, the surgery can be reduced to segmental reconstruction, which preserves the joint and physis (Fig. 1). Preservation of the adjacent natural joint is functionally beneficial [11], while preservation of the growth plate reduces gradual postoperative limb length discrepancies.
Representative indication for use of a 3-dimensional (3D)-printed custom-made implant. The plain radiograph and gadolinium-enhanced T1-weighted magnetic resonance image shows chondrosarcoma of the right distal femur. Anticipated surgical results are shown as segmental reconstruction using 3D-printed custom-made implant (right upper) and arthroplasty using a conventional tumor prosthesis (right lower).
To summarize the medical application of 3D printing for the treatment of bone tumors, this review focuses on the current clinical applications by usage type. The 3D-printing technology is being applied to bone-tumor surgery in various ways, such as surgical planning through preoperative bone-tumor modeling, fabrication of bone-replacing implants, and personalized surgical instruments [12]. To enhance understanding, here we present full treatment flows for young patients with malignant bone tumors who underwent limb salvage surgery.
3D printers and materials in orthopedic surgery
The International Standard Organization defines 3D printing as the “fabrication of objects through the deposition of a material using a print head, nozzle, or another printer technology.” In other words, 3D printing is an AM process that involves layerby-layer stacking. The main methods are based on powder solidification, liquid solidification, and extrusion [6]. Each 3D printer type has its own advantages and disadvantages, and the appropriate materials for printing are limited.
For clinical application in orthopedics, a few types of 3D printers with appropriate properties are commonly used. For bone modeling, various polymers or plaster can be utilized by any type of 3D printer if only a certain strength and precision are obtained. It is technically demanding to implement textures in simulated surgery, distinguish colors for selective anatomical structures, or use transparent materials. However, 3D-printed models, which are made of simple and single materials, are often sufficient in orthopedics. Unlike modeling, for surgical instruments or implants that are in contact with or placed in the surgical field, materials and 3D printers are subjected to strict regulations and certifications. Biocompatibility and sterilizing properties are important for their use as materials for surgical guides, the so-called patient-specific instrument (PSI). Biocompatible resins such as MED610, nylon, and polyamide have been utilized for PSI [13,14]. The fabrication of 3D-printed custom-made implants is limited by stricter regulations than those for PSI. Titanium alloy is the most widely used material for producing custom-made implants by 3D printing. Metal printing for medical use is usually of the powder-based type, and common printer types include electron beam melting and selective laser melting, whose energy delivery vary [15-22]. In the field of bioprinting and tissue engineering, 3D printing is still in the experimental stage, and various standard or custom-made 3D printers are used.
Clinical application in orthopedic oncology
1. Bone and tumor modeling
In many medical fields including orthopedics, visualization using a 3D-printed model is an easy and simple method. Before arthroplasty for severely destructive or deformative joints, bone modeling provides direct visualization of bone defects or deformities and opportunities for simulated surgery, including bone reaming and implant sizing and positioning [23-27].
In orthopedic oncology, 3D-printed models are also used for direct visualization via the creation of bone and tumor models [13,28,29]. However, virtual modeling is more common than real 3D-printed models and is utilized to design PSI and custom-made implants (Graphical abstract) [18-20,22,30].
2. Patient-specific instruments
In orthopedics, traditional cutting blocks or surgical guides for arthroplasty are non-patient-specific multiuse instruments. Many commercial PSI are utilized to enhance bone-cutting accuracy and reaming for arthroplasty. The PSI for conventional arthroplasty implants has a patient-specific shape but match each company’s commercial implant. However, in orthopedic oncology, PSI are utilized to ensure the accuracy of wide excision or fitting 3D-printed custom-made implants to bone defects. Implant fitting for massive bone defects is possible under the assumption of planned bone cutting. Therefore, the PSI in orthopedic oncology is designed with a 3D-printed implant.
Bone-cutting accuracy is the most important outcome of guide use. This often-reported problem is a major pitfall in PSI for arthroplasty [31]. Compared to other orthopedic surgeries, limb salvage surgery has advantages in this respect. Limb salvage surgery for sarcomas generally requires greater surgical exposure than other types of orthopedic surgeries. A wide opening in the surgical field can provide clear visualization of the bony landmarks and a sufficient contact surface of the PSI to the bone, thus reducing the possibility of PSI malpositioning. Bone-cutting accuracy by a PSI in orthopedic oncology is compatible to navigation system use [32], and Park et al. [14] reported maximal bone-cutting errors using a 3D-printed PSI of 3 mm and mean shortest and longest bone-cutting errors of 1.2 mm and 1.4 mm, respectively, of normal bones surrounding the tumor. Considering that the planned normal bone length surrounding the bone tumor is usually set to 1–2 cm or more in the wide excision of bone tissue, an error of even several millimeters may not adversely affect the oncologic margin status. Depending on the situation, the contact surface can be easily expanded with the closed-type PSI, or it is sometimes advantageous to deliberately use the open-type PSI. Notably, it is more important to fabricate a PSI while avoiding contact with soft-tissue expansion of the tumor or tough soft tissues that are difficult to remove (Fig. 2) [14,17].
3. Custom-made implants
Limb salvage surgery includes wide excision for bone sarcoma to enable bone and soft-tissue reconstruction. Wide excision for bone sarcoma often results in large bone defects, which have unique features in each patient. In other words, anatomical location, bone defect size and shape, adjacent joint involvement, and soft-tissue loss around the bone defect differ and require a personalized reconstruction strategy. One of the important aspects of limb salvage surgery is bone defect filling. Commercially available megaprosthesis is the most commonly used surgical option, but implant size and the applicable anatomical location are limited, and it is often difficult to use in children due to size mismatch. Structural bone allografts and recycled autografts are feasible options, but weak mechanical strength and osteolysis are major problems over time.
Metal 3D printing recently became feasible, and 3D-printed implants have been utilized to reconstruct massive bone defects after wide excision [12,15-22]. The major advantages of using 3D-printed implants are their “custom-made” nature. The concept of custom-made implants is not new, as these prostheses have been used reported for pelvic reconstruction until just recently. However, long fabrication times and high expense are major limitations [33,34]. As 3D-printing technology overcomes these limitations, custom-made implants are re-emerging. Regardless of bone and tumor size and location, implants can be fabricated in a few weeks by 3D printing. Moreover, unlike the previous custom-made implant that simply focuses on filling bone defects, 3D-printed custom-made implants attempt functional reconstruction by hybridization with conventional orthopedic implants or providing scaffolds for tissue integration [19,21]. When bone tumor occurs close to the joint, the joint is often sacrificed for conventional tumor replacement implants for implant fixation because of the short normal bone stock. However, using custommade implants, it is possible to save the joint that otherwise is lost due to being close to the bone tumor. Moreover, surgical time may be reduced compared to those other reconstruction options because 3D implants are already matched with the bone defects; therefore, processes such as allograft shaping can be omitted, and implant fixation is simply performed as planned without requiring processes such as plate bending. A direct comparison of the surgical time was not reported because of the variety of bone-tumor surgeries. For example, for bone-tumor surgery of the same pelvic bone, the surgery time may range from less than 2 hours to more than 10 hours depending on tumor size, surgical extent, bone reconstruction method, and artificial joint inclusion status. Park et al. [20] reported that reconstruction during limb salvage surgery using a 3D implant took 15–220 minutes, while the pelvic and long bones took 81–108 minutes, respectively. Liang et al. [18] and Wang et al. [35] reported 258 and 271 minutes for the whole procedure, including wide excision and reconstruction, respectively. The reconstruction time appears to have been shortened using 3D implants. Using 3D printed implants, surgeons can focus more on complete tumor resection by reducing the burden of reconstruction. Instead, it is necessary to spend significant preoperative time printing an optimized design and implant.
Clinical outcomes and complications after limb salvage surgery using custom-made 3D-printed implants have been reported in a few studies owing to the novelty of the 3D-printing technique. To date, several case series have reported promising surgical outcomes. Anatomical location seems to be the most important factor in terms of complications. The complication rate was reportedly high for pelvic reconstruction; in fact, it was the same as that of conventional implants. Compared to limb salvage surgery using conventional implants, 3D-implant surgery does not cause unexpected new complications or a high complications rate. However, long-term results are needed to properly evaluate clinical outcomes and complications (Table 1) [15,18-20,35-39].
Concerns about 3D implants are longevity related to the mechanical properties of the metal products made by a new fabrication method, metal powder-based AM. Clinical evidence for implant longevity is limited in previous studies due to the short follow-up period [15-20,22]. A solid 3D-printed structure made of Ti6Al4V has equivalent ultimate tensile strength, yield strength, and elongation [7,40]. Its fatigue properties, which are related to its surface roughness, unwanted porosity-related defects, building orientation, postprocessing such as machining, and hot isostatic pressing, require further study [41]. The mesh structure refers to a form with intentional internal porosity with a repeating pattern that can be fabricated only by AM. A mesh structure is utilized as a part of the implant body in orthopedic oncology and as a surface coating of cementless knee and hip arthroplasty implants [42,43]. This provides a scaffold for tissue integration and reduces the stress-shielding effect. However, the mesh structure is mechanically weak, and an implant fracture may be triggered at the interface between the solid and mesh structures [21].
4. Tissue engineering
Tissue engineering in orthopedic surgery is currently experimental. Skin, vessels, cartilage, and bone tissue are common targets in orthopedics [5,44-49]. In addition, bioprinting has advantages over traditional methods for 3D cell culture for creating and utilizing a tumor model [8].
Case presentation
1. Preoperation
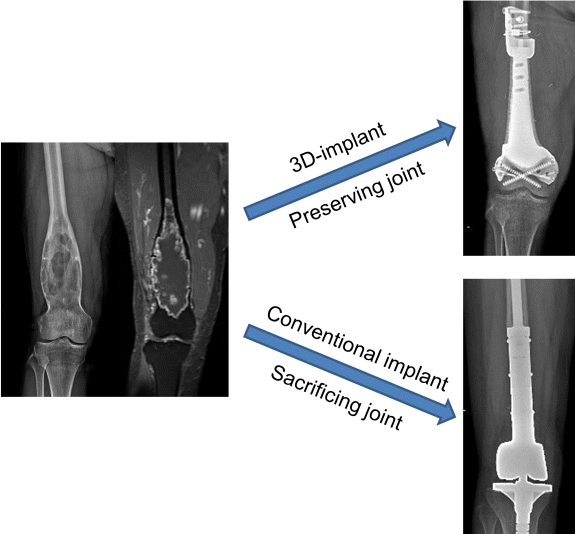
A 14-year-old girl presented with left knee pain. Plain radiographs showed a bone tumor with a mixed pattern but no clear tumor margin. Magnetic resonance imaging (MRI) showed that the tumor was located over the medial epiphysis and metaphysis of the proximal tibia (Fig. 3). Positron emission tomography/computed tomography (CT) showed no distant metastasis. Two cycles of neoadjuvant chemotherapy were administered before limb salvage surgery was performed. During chemotherapy, a 3D-printed implant was designed for the proximal tibia.
Case presentation of a 14-year-old girl with osteosarcoma of the proximal tibia. A plain radiograph (A) and T2-weighted magnetic resonance image (B) showed typical osteosarcoma of the proximal tibia. Photographs of the gross specimen (C) and a cross-section of the specimen (D) showing that a wide excision was completed as planned with sufficient margins. A postoperative plain radiograph (E) and computed tomography reconstruction image (F) presented bone reconstruction with the 3-dimensional-printed implant. The arrow indicates callus formation.
2. Design and manufacturing of 3D-printed implant
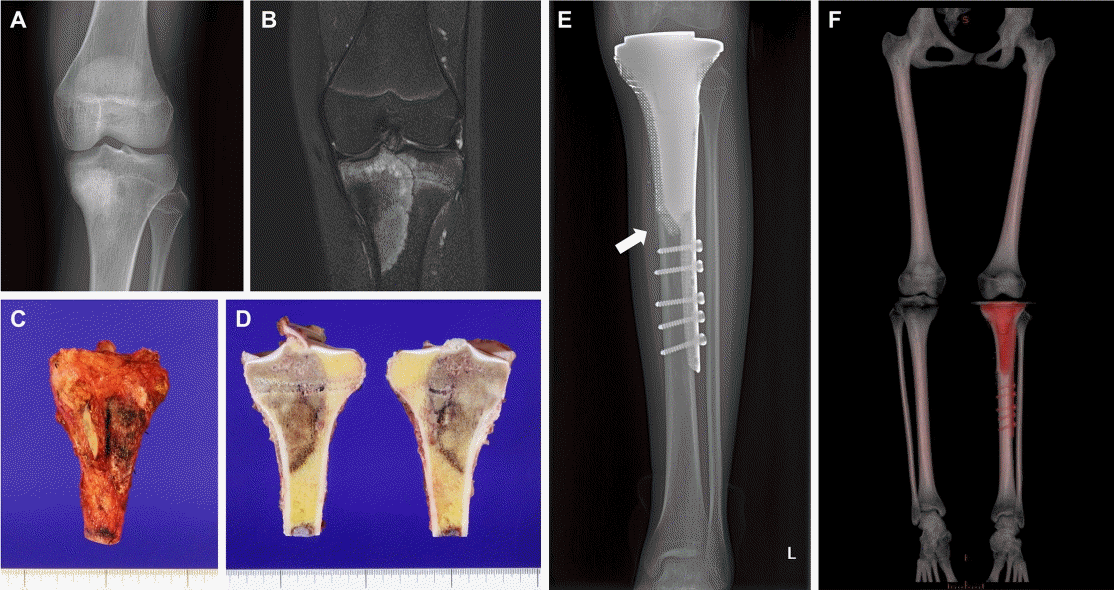
CT and MRI images of the proximal tibia were used to construct a 3D bone and tumor model using commercial bone modeling software (MIMCS, Materialize, Belgium). For the specific implant design, an orthopedic surgeon and an engineer are required to contribute and communicate. In terms of clinical considerations, we attempted to maintain the distal femur with growth potential and planned hemiarthroplasty for the proximal tibia only. We planned to insert only the femoral component in a future revision total knee arthroplasty. Intra-articular tumor invasion was not observed, and the distal bone margin for wide excision was set at 3 cm. PSI were designed to achieve the planned margins. To articulate, a conventional total knee arthroplasty implant was utilized in conjugation with a 3D-printed custom-made implant for the proximal tibia. The 3D-printed implant had a shape complementary to that of the conventional knee arthroplasty implant, with multiple holes for proximally suturing the patellar tendon and knee joint capsule. At the distal end of the implant, the implant body was transformed into a lateral plate for screw fixation of the distal tibia. The final implant was 196 mm long and weighed 153 g (Fig. 4).
Designs of the 3-dimensionally (3D)-printed products. Three-dimensional designs and photographs for the 3D-printed personalized surgical instrument (A) and the implant (B) are shown. (C) A design and photograph for the proximal tibial surface shows a matching socket for the tibial implant of conventional knee arthroplasty. (D, E) A design and photograph showing the 3D implant conjugated with the conventional knee arthroplasty implant.
3. Surgery with 3D-printed proximal tibial implant
With the patient in the supine position, a curved incision was made from the distal thigh to the lower leg. The patellar tendon was detached from the tibial insertion, and the soft tissues around the proximal tibia were dissected with a safe margin. A knee joint capsulotomy was performed, the cruciate ligaments were cut, and the tibia was completely separated from the distal femur along with the medial and lateral menisci. A tibial shaft osteotomy was performed using bone-cutting guides. The matched socket design of the conventional arthroplasty implant enabled simultaneous conjugation of the 3D-printed implant and tibial plate outside the surgical field during surgery. A 3D-printed custom-made cone-shaped reamer was used to enhance the bone-implant contact surface. The assembled implant (the 3D-printed implant and the tibial component of the conventional total knee arthroplasty implant) was placed in the bone defect, and the fitting was grossly perfect. The knee joint capsule and patellar tendon were sutured using premade suture holes in the 3Dimplant. Radiation exposure was minimized because the 3D-printed cutting guide eliminated the need for C-arm fluoroscopy during surgery.
4. Postoperative course
No adverse events occurred during the postoperative period. The suction drain for the surgical site was removed at 1 week postoperative, and intravenous antibiotics were continued over time. Adjuvant chemotherapy for osteosarcoma was resumed at 3 weeks postoperative. From 1 week postoperative, continuous passive knee motion was allowed and gradually increased to 90 degrees, and crutch walking with a knee brace was started. At the last follow-up 9 months after the index surgery, the patient was able to walk unsupported without limping (Fig. 4).
Conclusion
Three-dimensional printing technology is more actively utilized in orthopedic oncology than in other orthopedic fields. The 3D-printed implant can replace the conventional tumor prosthesis and auto/allobone graft, enabling personalized bone reconstruction. Currently, only a single titanium alloy material is practically used, but biodegradable materials or bioprinted materials will become available. Thus, biologic reconstruction using 3D techniques will be possible in the future.
Ethical consideration
The study was conducted after approval was obtained from the Institutional Review Board of National Cancer Center (NCC2017-0129). The presenting patient provided written informed consent prior to undergoing surgery after a thorough explanation of the surgical options.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by the National Cancer Center Grant (No. NCC-2110270).
Author contribution
Conceptualization: PJW, KHG; Data Curation: Not applicable; Formal analysis: Not applicable; Funding acquisition: PJW, KHG; Methodology: PJW, KHG; Project administration: PJW, KHG; Visualization: PJW, KHG; Writing-original draft: PJW; Writing-review&editing: PJW, KHG