Reemergence of mumps
Article information
Abstract
The mumps virus is a single-stranded, non-segmented, negative-sense RNA virus belonging to the Paramyxoviridae family. Mumps is characterized by bilateral or unilateral swelling of the parotid gland. Aseptic meningitis is a common complication, and orchitis is also common in adolescents and adult men. Diagnosis is based on clinical findings, but because of high vaccination coverage, clinical findings alone are not sufficient for diagnosis, and laboratory confirmation is needed. Mumps is preventable by vaccination, but despite high vaccination coverage, epidemics occur in several countries, including Korea. Many hypotheses are suggested for these phenomena. In this review, we investigate the reason for the epidemics, optimal methods of diagnosis, and surveillance of immunization status for the prevention of future epidemics.
Introduction
The history of mumps dates back to the 5th century B.C., when Hippocrates described it as "bilateral or unilateral swelling near the ears, and some of them had bilateral or unilateral pain and swelling of the testicles.". However, isolation and culture of the virus was only possible in 1945, and vaccination was first licensed in 1967. Without routine immunization, the incidence is estimated to be 100-1,000 cases per million, with an epidemic every 4-5 years. However, universal vaccination has contributed greatly to the decline in the incidence of mumps worldwide. Finland was the first country to declare itself mumps-free in 2000 after a national 2-dose MMR vaccination program for children, resulting in high vaccination coverage1). In Korea, the mumps vaccine was included in the National Immunization Program (NIP) in 1985, and a booster dose was given from 1997.
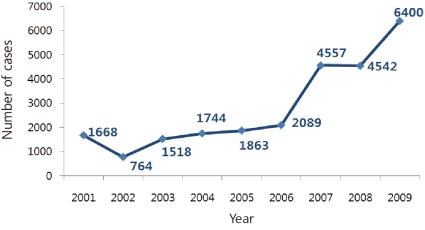
The clinical diagnosis of mumps is not difficult in population with low vaccination coverage, but currently because of high vaccine coverage and low incidence of mumps, clinical diagnosis of mumps is complicated. Major outbreaks have occurred worldwide, despite the high rate of vaccination coverage2-5). The incidence of mumps is on the rise in Korea, as reported by the Korea Centers for Disease Control and Prevention (KCDC) (Fig. 1). Various hypotheses have been suggested as the cause for the increase in mumps. The current status quo of mumps infections in Korea is described and potential preventive measures are suggested along with a review of the literature.
Virology
The mumps virus is a single-stranded, non-segmented, negative-sense RNA virus that belongs to the Paramyxoviridae family along with parainfluenza virus, measles virus, respiratory syncytial virus, and human metapneumovirus. It is composed of 7 major proteins, and the major antigens are glycoproteins V antigen and F protein, and a nucleocapsid protein S antigen. It is subdivided into 12 genotypes according to the hydrophobic membrane-associated protein (SH), and the B, F, and I genotypes are common in Asia. The cross-neutralization capacity of the genotypes is known to have decreased but this alone does not explain the increase in vaccine failure6), which needs to be addressed further. In terms of immunology, mumps has only 1 immunotype similar to the measles virus and humans are its only host. Its virulence is destroyed with heat and attenuated with UV light.
Epidemiology
Prior to vaccination, mumps was an epidemic disease, with a cycle of 4-5 years. It was predominant in the pediatric population, with seasonal outbreaks in winter and spring. It had been reported that by adolescence, 90% of the population demonstrated serologic evidence of infection7). Mumps was a well-known cause of aseptic meningitis and sensorineural hearing loss. The first dose of vaccination decreased the incidence of mumps by 88-98%, and the booster dose decreased the incidence by 97-99%8).
Sporadic outbreaks have been reported in several countries, including Belarus, the United States, Canada, the United Kingdom, Israel, Moldova, and Netherlands3-5, 9-12). In Korea, an outbreak was reported between 1986 and 1991 after the NIP was initiated, and the outbreak was believed to have been caused by primary vaccine failure. Subsequent administration of a booster dose contributed to a decrease in the incidence of mumps. Currently, in Korea, the incidence of mumps is on a constant rise, with 1,668 cases being reported in 2001, 1,518 cases in 2003, 4,557 cases in 2007, 4,542 cases in 2008, and 6,400 cases in 2009 (Fig. 1). The genotypes circulating in Korea have been identified as H and I13, 14).
The recent mumps outbreaks in the United Kingdom in 200515) and in the United States in 20069) were hot topics in the field of infectious diseases. MMR vaccination was initiated in the United Kingdom in 1988, followed by a booster dose in 1996. Such policies were helpful in decreasing the incidence of mumps to less than 5,000 cases in 2003. However, a sudden rise in the incidence of mumps was observed in 2005, with over 56,000 cases being reported in the United Kingdom. Most patients were 19-23 years of age and had only been vaccinated once.
An outbreak in the United States was reported in 2006; over 5,800 cases were reported. Most cases were in college students aged 18-24 years and the majority had been vaccinated twice16). Both effectiveness and uptake appear to not to have been sufficient to obtain herd immunity for mumps in populations such as college students17). A difference in the geometric mean titers (GMTs) of the serum neutralizing antibody in the causative strain and the Jeryl-Lynn strain used in the vaccine was also observed6). The epidemic occurred despite high vaccination rates and low mumps activity in the community18). Reports of outbreaks are continuing, with an outbreak in New York and New Jersey and also in highly vaccinated populations between June 2009 and January 201019).
The outbreaks are believed to be caused by multiple factors: inadequate levels of vaccination, primary or secondary vaccine failure, antigenic differences between the outbreak and vaccine strains, increased risk of transmission associated with college campuses, inherent limitations in mumps protective immunity, and misdiagnosis of infection.
Clinical manifestations
Some of the following clinical features may be found in cases of mumps.
1. Short course with non-specific signs and symptoms
2. Full-blown case with swelling of salivary glands without but no complications
3. Severe mumps and complications
4. No apparent symptoms but typical antibody responses
5. Meningoencephalitis or orchitis but no involvement of the salivary glands
Approximately 75% of all cases of apparent mumps in children are full-blown but without complications. Up to 30% of children infected with mumps may not even show signs of parotitis. Parotid gland swelling is at its maximum at 1-3 days and gradually decreases by 7 days. Generally, the clinical manifestations appear to be mild in children and severe in adults. Complications are less common after vaccination.
Except for the cases with complications, a typical mumps infection follows the course of fever, anorexia, headache, vomiting, and generalized aches and pains during the prodromal periods of days 1 and 2. Then, the parotid gland begins to enlarge and the enlargement is accompanied by slight redness of the orifices of the Stensen or Wharton ducts; 70-80% of cases show bilateral involvement.
Aseptic meningitis is the most common complication in children. Meningeal irritation signs are more prevalent in older children, adolescents, and adults, and nonspecific findings such as drowsiness and lethargy prevail in younger children. Further, 50-60% of patients have pleocytosis in the cerebrospinal fluid (CSF) but only one-sixth of these patients have meningeal symptoms. Encephalitis develops in approximately 0.5% cases, and mumps meningoencephalitis is known to have better prognosis than other viral meningoencephalitis.
Epididymo-orchitis and oophoritis does not occur if the infection occurs prior to adolescence. They are the most common clinical manifestations after parotitis in adolescent boys and adult men, usually in those aged 15-29 years. In 80% of all mumps orchitis cases, symptoms are first observed during the first 8 days of involvement of the salivary gland, usually unilateral involvement, with 15-30% of cases showing bilateral involvement20, 21). Sterility may be caused by bilateral orchitis in rare cases. Oophoritis occurs in approximately 7% of postpubertal female patients and manifests as pelvic pain and tenderness.
Pancreatitis occurs in 3% of cases22) but rarely with severe involvement that causes epigastric pain and tenderness, fever, chills, vomiting, and prostration. A connection with diabetes mellitus is suspected in experimental animals, which showed hyperglycemina and histologic lesions of the pancreatic islets.
Viruria is common in uncomplicated mumps cases and is accompanied by mild abnormalities in renal function23); severe and fatal nephritis is a rare complication.
Deafness occurs in 0.5-5.0/100,00 cases of mumps24), but mild degrees of hearing impairment are thought to be more common. Mumps-associated deafness may occur with or without meningoencephalitis after asymptomatic infection, and it is usually unilateral and often permanent. Occasional reports of vertigo have been reported.
Prior to the development of the vaccine, the incidence of mumps during pregnancy was 0.8-1.0 cases per 10,000 pregnancies25). An increase in fetal mortality in the 1st trimester, with a spontaneous abortion rate of 25% was observed but no evidence of in increased risk for fatal malformations was found26).
Other less frequent manifestations are exanthem and enanthem, arthritis, myocarditis, thrombocytopenia, keratouveitus, lower respiratory tract infection, and other glandular involvement (thyroiditis, mastitis, dacryoadenitis, and bartholinitis).
Transmission
The virus replicates within the upper respiratory tract and is transmitted through contaminated respiratory droplets or saliva and fomites. The virus is isolated 7 days before and 9 days after parotid gland swelling, with the greatest transmission during the 7-day period beginning 2 days before the onset of parotitis. Asymptomatic patients may also shed the virus. The incubation period spans from 12 days up to a maximum of 25 days and is usually known to be 16-18 days. Since the infectivity of mumps is lower than that of measles, a significant number of persons pass through childhood without being infected with the mumps virus.
Pathogenesis and pathology
Primary viral replication occurs in the upper respiratory mucosal epithelium. Then, the virus drains to the local lymph nodes. After that, viremia occurs, and the virus spread to multiple secondary infection sites, including the salivary glands, inner ear, pancreas, heart, nervous system, joints, kidneys, liver, gonads, and thyroid.
In response to viral infection, humoral and cellular immune responses are activated. The humoral immune response is induced by the production of serum antibodies to the V, F, and S antigens. The antibody to the S antigen persists for 3-7 days after the onset of symptoms and is short-lived; it is usually absent after 6 months. The antibody to the V antigen is noted for 2-4 weeks after the onset of illness and persists for long periods after infection. IgM is present early in the course of infection and is usually undetectable for 3 months after the onset of illness. IgG is detected at the end of the first week of illness; it peaks 3 weeks later and persists throughout life, mainly as IgG1. Salivary IgA is produced as well. Reinfection is common and patients with a history of mumps show antibody response patterns suggestive of reinfection and not primary infection. Specifically, the IgG but not IgM titer rises in these patients. The cellular immune response is also induced. In a study by Gans et al27), approximately two-thirds of vaccinees who did not seroconvert developed T cell immunity to the mumps virus. Jokinen et al28) reported very long persistence of vaccine-induced anti-mumps virus cellular immunity. Nevertheless at present, it is not known whether the humoral or cellular immune response is more important for protective immunity to the mumps virus.
Diagnosis
According to the Centers for Disease Control and Prevention (CDC), a clinical case of mumps is defined as acute onset of unilateral or bilateral tender, self-limited swelling of the parotid or other salivary glands that lasts for ≥2 days and occurs in the absence of other apparent causes. Confirmed cases are either laboratory confirmed or meet the clinical case definition and are epidemiologically linked to a confirmed or probable case. Probable cases meet the clinical case definition but are neither laboratory-confirmed nor epidemiologically linked to another confirmed or probable case. During epidemics, diagnosis may be made on the basis of history of exposure, incubation period of 2-3 weeks, and typical clinical manifestations of fever and parotitis. However, in sporadic cases or cases of mumps in a previously vaccinated child, laboratory findings such as virus isolation, virus detection, elevation of antibody titer, and serum amylase levels are needed to make a diagnosis. These criteria apply especially to cases occurring in areas with high vaccination rates.
Virus isolation is performed using the saliva, CSF, or seminal fluid collected within the first week of manifestation of symptoms. Virus isolation from blood is only possible during the first 3 days of illness. Cell culture, RT-PCR, and quantitative real time RT-PCR are used to detect the mumps virus. Culture as the sole method of diagnosis is not recommended since the cytopathic effect may not be evident in some strains of the mumps virus, and cellular pathologic changes may also be observed in other diseases that need to be differentiated from mumps. RT-PCR is more sensitive than cell culture-based methods, and quantitative real time RT-PCR may be needed to quantify the viral burden.
Serologic testing may be used to detect IgM, and this method is optimal for 7-10 days after symptoms develop. However, this method should be used with caution since those with a history of mumps or those who have been vaccinated may not demonstrate a rise in IgM levels. IgM is not detected after 3 months. IgG is first detected 1 week after symptoms manifest, and its titer peaks at 3 weeks after initial symptoms. However, vaccination may prevent such peaks and hence should not be used to exclude the diagnosis of mumps. Serologic testing is only informative for mumps diagnosis and is of limited predictive value for assessing protecting immunity.
Differential diagnosis
The mumps virus is not the only cause of parotitis. Parotitis may be caused by EBV, coxsackieviruses, echoviruses, influenza A virus, parainfluenza viruses, CMV, HHV-6, etc. Bacterial parotitis is characterized by exquisite tenderness of the region, elevated WBC count, and pus draining from the Stensen duct. Lymph node enlargement is also an important differential diagnosis. Clinical findings need to be supplemented with laboratory evidence for the diagnosis of mumps, but only 10% of clinically diagnosed mumps could be confirmed with laboratory tests29). A change in the diagnosis after serologic testing in a patient suspected to have mumps is common in clinical settings30).
Treatment
There is no specific treatment for mumps. Symptomatic treatment such as hydration and alimentation of patients is important, and analgesics may be used to treat the severe headaches or discomfort caused by parotitis. Antiviral agents are inappropriate and not indicated. Supportive treatment should be provided in cases with mumps orchitis. Steroid administration to patients with mumps orchitis is not recommended because steroids may further lower the level of testosterone and increase the level of FSH and LH, further aggravating atrophy of the testes31, 32). Postinfectious encephalitis, Guillain-Barré syndrome, and idiopathic thrombocytopenic purpura (ITP) are reported as autoimmune-related diseases that occur after mumps, and these may be treated with IVIG33-35).
Prognosis
Symptomatic treatment aids greatly in recovery in most cases. Meningoencephalitis has a generally favorable outcome, although neurological damage and death can occur. Deafness and sterility are rare complications.
Immunization
Mumps can be prevented by the measles, mumps, rubella (MMR) vaccine. The MMR vaccine is widely used worldwide (in 114 nations) since being licensed in 1967. The first dose is given at 12-15 months of age, and the timing of the second dose differs among countries. Vaccination of 90% of the population is believed to provide herd immunity against the mumps virus36). As with rubella, insufficient childhood vaccination coverage against mumps can result in an epidemiological shift in disease incidence to older age groups, potentially leading to higher rates of serious disease and complications than that before large-scale immunization was introduced. Therefore, mumps vaccination as part of a NIP should entail aiming for a high level of disease control.
Viral strains used in the vaccines include Jeryl-Lynn, RIT 4385, Urabe Am9, Leningrad-3, and Leningrad-Zagreb strains. There are 2 types of vaccines currently marketed in Korea. One is Priorix® from GSK; it uses the RIT 4385 strain. The other is MMR® II from Merck; it uses the Jeryl-Lynn strain. The Rubini strain was abandoned in routine vaccination programs by the WHO because of its low efficacy37). The Urabe AM9 strain was withdrawn from the market because it caused aseptic meningitis in February 2002 in Korea.
Further, 95% efficacy was reported with the Jeryl-Lynn strain in randomized clinical trials38, 39), but the actual clinical efficacy during outbreaks was 61-91%5). Clinical efficacy during outbreaks were acceptable for all strains, except the Rubini strain5). These findings were confirmed not only in Europe and the United States but also in Singapore, an Asian country similar to Korea2). The reason for the decrease in clinical efficacy during outbreaks is thought to be because of less than optimum herd immunity in high-risk settings for exposures, improper storage of the vaccine, primary vaccine failure, secondary vaccine failure (waning of immunity), heterologous reinfection, confounding factors, selections bias, etc. In addition, the level of vaccine effectiveness may decrease with time after vaccination4). In a comparative study, the seronegativity rate after 4 years of vaccination was 15% for the Urabe strain and 19% for the Jeryl-Lynn strain40). Mumps seropositivity rates and antibody titers peaked shortly after the first and second MMR vaccinations and declined rapidly within a year after the first dose and at a slower pace after the second dose41). These findings suggest that waning immunity may contribute to the occurrence of mumps outbreaks in older vaccinated populations.
Post-exposure vaccination is not effective for mumps, although vaccination is needed to prevent further infections, and the risk does not increase even if vaccination is performed during the incubation period.
We cannot predict who will get mumps during an outbreak. No serologic test reliably predicts who is at risk and who is not42). Virus neutralization is the best test available3), and another valuable method is avidity testing for mumps virus-specific IgG43). However, there is no established mumps neutralizing antibody titer predictive of protection against infection or disease. The presence of mumps IgG is useful for diagnosis of mumps infection, not for predicting protection against mumps. Thus, the interval between vaccination and exposure is obviously important but is not the only factor influencing susceptibility during the recent outbreaks of mumps. In the absence of opportunities for antigenic boosting, mumps antibody titers will likely continue to wane, and greater numbers of individuals who are susceptible to mumps may eventually accumulate, resulting in outbreaks that occur less frequently but are more severe than previous outbreaks.
It is important to consider that individuals possessing low antibody titers or even those lacking measurable antibody titers might not necessarily be susceptible to symptomatic mumps virus infection because they might be fully protected by cell mediated immunity. Cell-mediated immunity has been observed in seronegative vaccine recipients even 21 years after vaccination28).
Conclusion
Despite the high coverage of immunization, mumps outbreaks are reported. Various factors are considered to explain this observation, such as primary or secondary vaccine failure, inadequate levels of vaccination, antigenic differences between the outbreak and vaccine strains, increased risk of transmission associated with college campuses, inherent limitations in mumps protective immunity, and misdiagnosis. To prevent major outbreaks, further investigations are needed to identify the optimal method of diagnosis, monitor immunization status, and establish the most efficient vaccination schedule. However, as observed in the Finland outbreak, maintaining a high vaccination rate is the most important step toward preventing a mumps outbreak.