Disease-specific pluripotent stem cells
Article information
Abstract
Induced pluripotent stem (iPS) cells are generated by epigenetic reprogramming of somatic cells through the exogenous expression of transcription factors. Recently, the generation of iPS cells from patients with a variety of genetic diseases was found to likely have a major impact on regenerative medicine, because these cells self-renew indefinitely in culture while retaining the capacity to differentiate into any cell type in the body, thereby enabling disease investigation and drug development. This review focuses on the current state of iPS cell technology and discusses the potential applications of these cells for disease modeling; drug discovery; and eventually, cell replacement therapy.
Introdction
Since their first isolation, embryonic stem (ES) cells have been regarded as the gold standard for potential use in cell-based regenerative medicine. The pluripotent nature of these cells provides them with, the ability to self-renew indefinitely in culture while retaining the capacity to differentiate into any cell type in the body1). However, the fact that ES cells can only be derived from early-stage embryos precludes the establishment of autologous cell lines for patients and immune rejection hinders the use of nonautologous ES cell lines2).
The advent of mammalian somatic cell nuclear transfer (SCNT) first appeared to offer solutions for overcoming these roadblocks. SCNT is a technique whereby the DNA of an unfertilized egg is replaced by the DNA of a somatic cell. Although significant advancements toward this goal have been made, successful SCNT into human cells remains an elusive goal and is fraught with social and logistical concerns3). Alternative methods for deriving pluripotent cells, such as cell fusion and culture-induced reprogramming, have been developed, but these approaches still suffer from severe practical and technical limitations4).
In 2006, Takahashi5) and Yamanaka reported the astonishing discovery that somatic cells could be reprogrammed to adopt the pluripotent stem cell state. The generation of pluripotent cells by exogenous expression of transcription factors circumvents many previous limitations because this approach is not technically demanding and does not require embryonic material or oocytes. We therefore believe that this discovery which is now known as "induced pluripotent stem cell technology" (iPS) will have a significant impact on regenerative medicine. In this article we review the current methodologies used for generating iPS cells and then discuss their potential clinical applications.
Reprogramming somatic cells towards pluripotency by defined transcription factors5)
To establish the transcription factors necessary for reprogramming of differentiated cells, mouse embryonic fibroblasts and tail-tip fibroblasts of mice were initially used. Reprogrammed somatic cells were identified by selecting for the reactivation of the Fbx-15 gene locus with a neomycin-reporter cassette whose expression is restricted to the early stage of embryonal development. Cells which are only resistant to G418 selection allowed growth of ES-cell-like colonies and cells derived by sub-culturing these ES-like colonies were designated as "induced pluripotent stem cells." It was found that of the 24 candidate genes that are implicated in the establishment and maintenance of the pluripotent state, Oct4 (Pou5f1), Sox2, c-Myc, and Klf4, were sufficient to mediate reprogramming. These iPS cells had fully demethylated Oct4 and Nanog promoters and could contribute to the formation of germline-competent chimeras upon injection of blastocysts, a characteristic which demonstrates true pluripotency.
Preferred methods for preparation of iPS cells
First-generation iPS cells were generated by retroviral transduction5). Since then, the technique has been optimized and reproduced in a number of different processes6). Several important variables include the choice of cell type for reprogramming, the choice of the combination of genes and small molecules used for reprogramming, as well as the gene transfer method (Fig. 1).
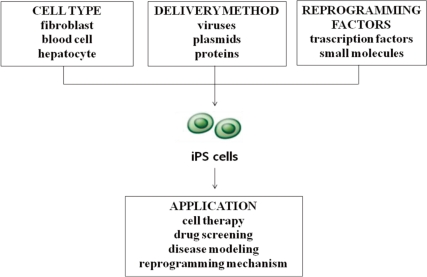
Overview of the processes for inducing the derivation of pluripotent stem (iPS) cells and clinical applications.
For the first reprogramming attempts of both mouse and human cells, fibroblasts were used as the starting cell population5). Since the original success of fibroblast reprogramming, multiple cell types have been used for reprogramming, including keratinocytes, neural progenitor cells, hepatocytes, stomach epithelial cells, pancreatic beta cells and intestinal epithelial cells7). Several factors must therefore be considered in determining the optimal cell type for a given application: (1) the ease with which reprogramming factors can be introduced, which varies according to cell type and delivery approach; (2) the availability and ease of derivation of the given cell type; and (3) the age and source of the cells used6). An inverse correlation has been effectively demonstrated for the differentiation stage of target cells. For example, with mouse hematopoietic stem and progenitor cells being more efficiently reprogrammed than terminally differentiated B and T lymphocytes8, 9). While fibroblasts are likely to remain the choice cell type in basic research efforts, iPS cells derived for therapeutic purposes will require the donor cells to be easily attainable, less likely to contain genetic aberrations, and easy to reprogram using transient approaches6).
Since the first report of reprogramming, it has become evident that there is some flexibility in the factors needed to reprogram somatic cells. Of the original four transcription factor-encoding genes, c-Myc is not strictly required for the reprogramming of somatic cells and Klf4 and Sox2 have been shown to be dispensable in reprogramming strategies utilizing cell types that endogenously express them. Oct4 is the only factor that cannot be replaced by other family members and the only one that has been required in every reprogramming strategy in either mouse or human cells8).
To aid in the production of epigenetic modifications during the reprogramming process, several studies have examined the use of small-molecule compounds. Importantly, small molecules, including DNA methyltransferase inhibitors10), the histone deacetylase inhibitor valproic acid11), and the histone methyltransferase inhibitor BIX-0 129412), have been shown to substantially improve the efficiency of reprogramming, even in cases without inclusion of exogenous Klf4 and c-Myc (for valproate) or exogenous Sox2 and c-Myc (for BIX-01294). In addition, inhibition of DNA methyltransferase activity by 5-aza-cytidine also was found to improve reprogramming efficiency13). Recently, another small molecule, transforming growth factor RI kinase inhibitor II, has also been reported to replace Sox2 by the induction of Nanog14). Of note, the effects of these small-molecule agents are not directed towards specific reprogramming events, and it remains to be determined whether full reprogramming through the exclusive use of chemicals is possible.
iPS cells have been generated using a number of different gene transfer methods, including retroviral, lentiviral, and adenoviral vectors and nonviral plasmids, and recently by direct recombinant protein delivery8). Initial generations of mouse and human iPS cells employed retroviral vectors5) and constitutive lentiviruses15). These viral systems, however, have been criticized because they are permanently integrated into the genome and endeavors to make iPS cells more therapeutically applicable have led to the pursuit of non-integrative approaches. A refined system which employs the Crelox system or transposases in which reprogramming factors could be delivered into the host genome and removed without any residual elements has been recently developed16). More importantly, iPS cell generation has now been achieved without genomic integration. This has been accomplished using adenoviruses17), repeated plasmid transfection18), and recombinant proteins19) and via the use of episomal vectors20) in human cells. However, the reprogramming efficiency obtained using these delivery systems tends to be dramatically lower for most cell types relative to the standard retroviral/lentiviral mediated reprogramming7). Ultimately, the logistical, financial, and practical aspects of each technique must be taken into account. It is likely that the optimal cell reprogramming method will be determined by the intended application of the reprogrammed cells. Currently, for example, the fastest and most efficient method of creating human iPS cell lines through lentiviral and retroviral transduction might represent the preferred approaches for generating iPS cells for use in large-scale drug-screening programs and disease modeling8).
Clinical relevance
The recent introduction of iPS cell technology has opened a new avenue not only for cell replacement therapy but also for disease modeling and drug development21). The generation of human iPS cells from patients with a variety of genetic diseases with either Mendelian or complex inheritance including X-linked adrenoleukodystrophy (X-ALD), amyotrophic lateral sclerosis, Duchenne and Becker muscular dystrophy, Parkinson's disease, Huntington's disease, Gaucher disease type III, and Down syndrome is also possible. Such disease-specific iPS cells offer an unprecedented opportunity to recapitulate both normal and pathologic human tissue formation in vitro, thereby enabling disease investigation, drug development and cell therapy21).
Conclusion
As a result of its tremendous potential in a wide variety of clinical and research applications, there is great interest in cellular reprogramming. The recent successes in iPS cell derivation without the use of viral vectors and genomic integration from human cells has brought the realization of the therapeutic potential of iPS cell technology closer than ever. Importantly, however, there is still much to learn about how this reprogramming process works. The key steps involved in this process consist of the choice of reprogramming factors and molecules, their delivery method, and the choice of target cell type. Given the high level of interest in iPS cells and the large number of laboratories around the world that are now studying these cells, we are hopeful that iPS cell technology will have a positive impact on therapeutic interventions.