Effects of human growth hormone on gonadotropin-releasing hormone neurons in mice
Article information
Abstract
Purpose
Recombinant human growth hormone (rhGH) has been widely used to treat short stature. However, there are some concerns that growth hormone treatment may induce skeletal maturation and early onset of puberty. In this study, we investigated whether rhGH can directly affect the neuronal activities of of gonadotropin-releasing hormone (GnRH).
Methods
We performed brain slice gramicidin-perforated current clamp recording to examine the direct membrane effects of rhGH on GnRH neurons, and a whole-cell voltage-clamp recording to examine the effects of rhGH on spontaneous postsynaptic events and holding currents in immature (postnatal days 13-21) and adult (postnatal days 42-73) mice.
Results
In immature mice, all 5 GnRH neurons recorded in gramicidin-perforated current clamp mode showed no membrane potential changes on application of rhGH (0.4, 1 µg/mL). In adult GnRH neurons, 7 (78%) of 9 neurons tested showed no response to rhGH (0.2-1 µg/mL) and 2 neurons showed slight depolarization. In 9 (90%) of 10 immature neurons tested, rhGH did not induce any membrane holding current changes or spontaneous postsynaptic currents (sPSCs). There was no change in sPSCs and holding current in 4 of 5 adult GnRH neurons.
Conclusion
These findings demonstrate that rhGH does not directly affect the GnRH neuronal activities in our experimental model.
Introduction
The use of recombinant human growth hormone (rhGH) has been increased in a variety of conditions associated with short stature, such as growth hormone deficiency, Turner syndrome, chronic renal failure, Prader-Willi syndrome, sustained postnatal growth failure in children who have been small for gestational age, and idiopathic short stature1, 2). Many studies have shown that the administration of rhGH increases height2-5). However, there are some concerns that growth hormone (GH) treatment may induce skeletal maturation and an early onset of puberty. Randomized controlled studies for rhGH treatment of patients with idiopathic short stature have yielded differing results regarding the influence of GH on pubertal onset, pubertal pace, and bone maturation. Some authors demonstrated there was no effect on pubertal onset or pace in boys and girls6, 7), while others demonstrated there was an acceleration of pubertal onset and bone maturation8, 9).
There are data to support the role of GH in the control of reproductive functions and the initiation of puberty. The suppression of GH secretion in female rats has been shown to delay puberty10). In male rats, the experimental induction of GH deficiency is associated with the delay in testicular growth and the differentiation of the germ cells11), suggesting a role for GH in the induction of puberty. The puberty onset in the GH receptor gene disrupted mice as determined by the age at vaginal opening was delayed12). Plasma LH response to GnRH treatment was significantly attenuated in GH receptor gene knockout mice13). However, the majority of the physiologic effects of GH on puberty has been explained by action of IGF-I. Patients with Laron syndrome are infertile, and administration of IGF-I initiates puberty in these subjects14). Infusion of IGF-I has been shown to stimulate GnRH release from the median eminence and accelerate the onset of puberty in female rats15, 16). Zhen et al.17) demonstrated the expression of IGF-I receptors on GnRH neuronal cell line, and activation of GnRH mRNA expression and GnRH neuronal cell proliferation by IGF-I treatment. Although there are few studies suggesting GnRH neurons are activated by IGF-I, the direct effect of GH on GnRH neurons remains to be defined. In this study, we tried to elucidate the effect of rhGH on immature and adult GnRH neurons.
Material and methods
1. Experimental animals
All experiments were approved by Chonbuk National University Animal Welfare and Ethics Committee. GnRH-green fluorescent protein tagged mice (Transgenic GnRH-EGFP-mut5) were housed under 12-h light, 12-h dark cycles (lights on at 07.00 h) with ad libitum access to food and water18). Fifteen immature mice (postnatal day 13 to 21) and 14 adult mice (postnatal day 42 to 73) were used.
2. Brain slice preparation
Patch-clamp recordings were obtained in acutely prepared coronal slices as previously described19). Mice were decapitated and brains were rapidly removed and placed in ice-cold bicarbonate-buffered artificial cerebrospinal fluid (ACSF) of the following composition (in mM): 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.2 MgCl2, 11 D-glucose, 1.4 NaH2PO4 and 25 NaHCO3 (pH 7.3-7.4 when bubbled with 95% O2 and 5% CO2). Brains were blocked and glued with cyanoacrylate to the chilled stage of a vibratome (Microme, Walldorf, Germany), and 150 to 250 µm-thick coronal slices containing rostral preoptic area were cut. The slices were allowed to recover in oxygenated ACSF for at least 1 hour at room temperature.
3. Chemicals
Recombinant humane growth hormone (Eutropin®) was provided by LG Life Science, Ltd., and chemicals for ACSF and somatostatin were purchased from Sigma (USA). Applied reagents were dissolved in the ACSF solution and were diluted by 1,000 in perfused ACSF before use. The rhGH concentrations of ACSF solution were 0.2 µg/mL, 0.4 µg/mL and 1 µg/mL respectively based on the previous studies20-22).
4. Electrophysiology and data analysis
The coronal slices were transferred to the recording chamber, held submerged, and continuously superfused with carboxygenated ACSF at a rate of 4-5 ml/min. The slices were viewed with an upright microscope (BX51WI; Olympus, Tokyo, Japan) and fluorescent GnRH neurons identified at 10X and 40X objective magnification by brief fluorescence illumination and then viewed and patched under Nomarski differential interference contrast optics. Patch pipettes were pulled from thin-wall borosilicate glass-capillary tubing (PG52151-4, WPI, Sarasota, USA) on a Flaming/Brown puller (P-97; Sutter Instruments Co., Novato, CA). The pipette solution was passed through a disposable 0.22 µm filter and contained (in mM): 140 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 4 MgATP, and 10 EGTA (pH 7.3 with KOH) for the whole-cell experiment. The whole-cell patch-clamp recordings were were performed under voltage clamp using an Axopatch 200B (Axon Instruments, Union City, CA). The tip resistance of the electrode was 4-6 MΩ. The cells were voltage clamped at -60 mV after nullifying the potential between the patch pipette and bath solution. After a giga-seal, the membrane was ruptured by applying a slight negative suction. Membrane current changes were sampled online using a Digidata 1322A interface (Axon Instruments, USA) connected to an IBM PC. Any GnRH neurons that displayed a shift in holding current of >5 pA were considered to have responded. For perforated patch-clamp recording, a pipette solution containing (in mM): 130 KCl, 5 NaCl, 0.4 CaCl2, 1 MgCl2, 10 HEPES, and 1.1 EGTA (pH 7.3 with KOH) was used. Gramicidin (Sigma, St. Louis, USA) was first dissolved in dimethylsulfoxide (Sigma) to a concentration of 2.5-5 mg/ml and then diluted in the pipette solution just before use to a final concentration of 2.5-5 µg/mL and sonicated for 10 minutes. In initial experiments, access resistance was monitored and experiments were begun when resistance stabilized at 50-90 MΩ. This typically took 15-20 minutes after giga-seal formation and always corresponded to the resting membrane potential (RMP) of the cell reaching a stable level below -45 mV. Spontaneous rupture of the membrane was evident by a sudden overshooting of action potentials above 0 mV. Acquisition and subsequent analysis of the acquired data were performed using the Clampex9 software (Axon Instruments, USA). Spontaneous postsynaptic currents (sPSCs, recorded at -60 mV), were detected and analyzed using Minianalysis (Synaptosoft). To assess the effects of rhGH on amplitude and frequency, the traces were measured and analyzed in 3-minute epochs using algorithms provided by Mini Analysis software. Traces were plotted using Origin7 software (Micro Cal Software, Northampton, MA). All recordings were made at room temperature.
5. Statistics
All values were expressed as mean (SEM). The student Wilcoxon rank sum test was used to compare the means of 2 experimental groups. The Kruskal-Wallis test was performed to compare the membrane potential changes, and resting membrane potential (RMP), as well as the frequency and amplitude between more than 2 experimental groups. A level of P<0.05 was considered to be significant.
Results
Electrophysiological recordings were obtained from a total of 29 GnRH neurons. Fourteen neurons were recorded in gramicidin-perforated patch-clamp mode, and the other 15 neurons were recorded in whole-cell voltage clamp mode at a holding potential of -60 mV (Table 1). Under gramicidin-perforated patch, the mean RMP was -61.5±2.14 mV (n=14).
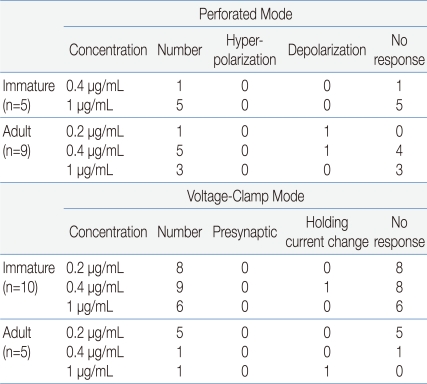
Summary of Responses to rhGH of GnRH neurons in Perforated Current Clamp and Whole-Cell Voltage-Clamp Modes
In gramicidin-perforated patch-clamp mode, none of the 5 immature GnRH neurons (mean RMP = -59.2±4.71 mV) showed response to applied rhGH (0.4, 1 µg/mL) (Fig. 1A). In adult GnRH neurons, 7 (78%, RMP = -60.2±2.59 mV) of 9 neurons showed no response to applied rhGH (0.2-1 µg/mL) (Fig. 1B) and 2 neurons showed weak prolonged membrane depolarization (Fig. 1C) (3.79±0.68 mV n=2, 14.3%).
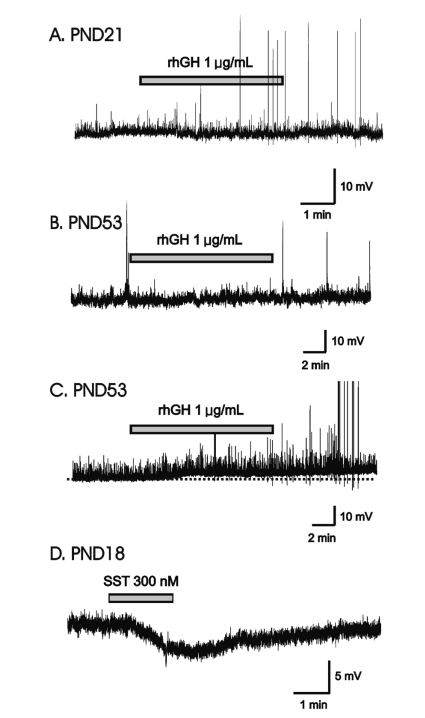
rhGH-induced membrane depolarization in a minority of GnRH neurons only in adult. (A) Gramicidin-perforated patch recording of GnRH neuron (PND21) showing no response to applied rhGH (1 µM). (B) Gramicidin-perforated patch recording from an adult GnRH neuron showing no response to applied rhGH (PND53). (C) Gramicidin-perforated patch recording from adult showing sustained depolarization by rhGH (PND53). Dotted line indicates base line. (D) Application of 300 nM somatostatin induced membrane hyperpolarization on a GnRH neuron (PND18). Abbreviations: rhGH, recombinant human growth hormone; GnRH, gonadotropin-releasing hormone; SST, somatostatin; PND, postnatal day.
We tested somatostatin (SST), a known neuromodulator peptide in the central nervous system which suppresses the release of growth hormone (GH) as an opposite control. When SST was applied in the same cell tested with rhGH, SST induced the clear and potent membrane hyperpolarization (Fig. 1D) suggesting SST may directly act on GnRH neurons and can suppress their activities.
In order to clarify whether the application of rhGH produces any change on postsynaptic current and holding current, 15 GnRH neurons were tested with rhGH (0.2-1 µg/mL) in voltage-clamp mode with a holding potential of -60 mV (Table 1). Four out of 5 neurons from adult mice showed no changes in holding current and postsynaptic activities with the influence of rhGH. Nine (90%) of 10 immature neurons showed no response to rhGH (Fig. 2A). Among 15 neurons, 8 neurons were tested for the dose dependent effect, a concentration ranging from (0.2-1 µg/mL) was applied, and none of them showed any change in synaptic events and holding current. Further postsynaptic currents were truncated to better depict changes in amplitude (Fig. 2B) and inter event intervals (IEE) (Fig. 2C). The rhGH did not shift the cumulative distribution histogram trace in any circumstances suggesting no change in amplitude and inter event interval upon application of rhGH. The amplitude and the frequency have shown no changes on application of rhGH (Fig. 2D, 2E). Five of 8 neurons applied with SST (300 nM) induced an outward current (15.8±0.52 pA) (Fig. 2F); however, the other 3 neurons showed no response.

rhGH induced no change in the holding current of GnRH neurons. (A) Voltage-clamp recording of a GnRH neuron (postnatal day 21) holding at -60 mV showing no response to applied rhGH. (B) Amplitudes of postsynaptic currents were truncated to better depict changes in holding current. (C) A cumulative distribution graph of amplitude and IEE before, on, and after rhGH application, was obtained from the same cell as in A. (D), (E) A cumulative bar graph showing frequency before (1.93±0.69), on (1.88±0.68), and after (1.74±0.52), and amplitude before (46.8±0.56), on (47.8±0.60), and after (47.1±0.60), application of postsynaptic currents from 7 immature GnRH neurons. No significant difference was found in the frequency and amplitude before, on, and after rhGH application. (F) Application of 300 nM somatostatin induced an outward shift in holding current on GnRH neuron (postnatal day 21). Abbreviations: Cont, control; IEE, inter-event interval; rhGH, recombinant human growth hormone; SST, somatostatin; WO, washout.
Discussion
With the development of recombinant DNA technique, the therapeutic use of GH is expanding not only to patients with GH-deficient short stature but also to children with non-GH-deficient short stature1, 2). Children with short stature are treated with GH under the assumption that there is no interference of GH treatment on the timing of puberty. However, the effect of GH therapy on pubertal onset and bone maturation of children remains controversial. Low dose GH6) and medium dose GH treatment7) had no effect on pubertal onset or pace. On the other hand, some studies suggest that GH treatment may induce an early onset of puberty and accelerated skeletal maturation. High dose GH treatment was associated with acceleration of pubertal onset and bone maturation8). Kaplowitz23) and Kawai et al.9) reported that GH therapy for boys did not have a beneficial effect on their final height because bone maturation was accelerated during GH therapy.
There have been reports on the role of GH in the control of reproductive functions and the initiation of puberty. Firstly, GH might exert a direct effect on the ovaries. GH receptor mRNA expression and GH binding protein (GHBP) been detected in the ovaries in humans24), and in several animal species25-29). GH treatment augments the follicle-stimulating hormone (FSH)-induced differentiation of ovarian granulosa cells30). Furthermore GH, in the absence of FSH, can induce folliculogenesis in isolated ovarian preantral follicles of immature mice, strongly suggesting a role of GH in the control of ovarian function31). In male rats, the experimental induction of GH deficiency is associated with the delay in testicular growth and the differentiation of the germ cells11). Secondly, GH has an effect on gonadotropes in the pituitary gland. Plasma LH response to GnRH treatment was significantly attenuated in GH receptor gene knockout mice13). GH-treated monkeys have an earlier initial rise in serum LH concentrations and secrete significantly higher amounts of estradiol compared to non-treated monkeys32). GH antigens are present in pituitary cells containing FSH or LH mRNAs and in cells containing GnRH receptors indicating that either GH cells are transitory gonadotrophs, or GH is present in these pituitary cells most likely assisting to control their function33, 34). In addition, GH-binding protein antigens were identified in pituitary cells that contained LH and FSH, indicating a possible paracrine effect of GH in the control of the gonadotrope functions35). Therefore, GH may function as a "co-gonadotropin"36). Thirdly, GH has an indirect effect via IGF-I on the ovary and GnRH neuron. Patients with Laron syndrome are infertile and it has been demonstrated that administration of IGF-I initiates puberty in these subjects14). It has been shown in prepubertal female rats that IGF-I increases GnRH release16). It is known that in addition to being derived from the peripheral origin, IGF-I is synthesized within the hypothalamus and the pituitary gland, and there is evidence for the presence of its receptors in the hypothalamus and the pituitary gland16). In vitro studies have revealed that IGF-I increases GnRH-induced gonadotropin secretion by the pituitary cells15). Zhen et al.17) demonstrated the expression of IGF-I receptors on GnRH neuronal cell line, and activation of GnRH mRNA expression and GnRH neuronal cell proliferation can be induced by IGF-I.
Recently, the effects of GH on the central nervous system (CNS) have received much attention. In humans, GH receptors are found in most areas of the CNS. They are detected in their highest concentrations in the choroid plexus, but are also found in the hippocampus, putamen, thalamus, pituitary and hypothalamus37-39). The GH receptor mRNA has also been detected in samples of human fronto-parietal and temporal cortex40). There are some reports that support the ability of GH to cross the blood brain barrier (BBB). In acromegalic patients with excessive GH levels in their plasma, some researchers have found normal GH levels in the cerebrospinal fluid (CSF)41), whereas others have found increased levels42). When GH is applied externally for GH substitution, there is an increase in GH levels in the CSF43, 44). In conformity with insulin and leptin, GH has been suggested to reach its responsive sites in the brain following a receptor-mediated transport across the BBB45, 46). Among all the effects exerted by GH on brain function, some may result from a direct action of the hormone on its brain receptors, whereas other effects elicited by the hormone may be due to GH-induced mediator-insulin-like growth factor-I (IGF-I). There have been some studies on the effects of IGF-I on GnRH neurons.
This study was undertaken to determine the direct effects of GH on GnRH neurons, key regulator of the reproductive system. Since GnRH neurons are distributed sparsely throughout the hypothalamus, the studying of living GnRH neurons has been complicated. With the development of transgenic mice in which green fluorescent protein is genetically targeted to GnRH neurons, studies of living GnRH neurons have been facilitated47). We performed a gramicidin-perforated patch- clamp study to elucidate the effect of rhGH on electrical activities of GnRH neurons. Gramicidin forms pores only permeable to monovalent cations48) and keeps the concentration of intracellular chloride unchanged by the pipette solution, and further preventing washout of intracellular factors that can be important for a second messenger system. Using perforated patched-clamp recordings, Han et al. demonstrated that GnRH exerts depolarizing actions on the excitability of GnRH neurons49) and kisspeptin evokes long-lasting depolarization of GnRH neurons in adult mice50).
Our findings demonstrate that 1) in gramicidin-perforated current clamp mode, the majority of GnRH neuron tested did not show any response to applied rhGH in varying concentrations; and 2) in whole-cell voltage-clamp mode, there was no significant change in holding current and postsynaptic currents. These results indicate that rhGH does not directly affect the excitability of GnRH neurons.
We tested limited numbers (n=29) of GnRH neurons which is a small population to represent whole GnRH neurons, and thus it is haste to conclude that rhGH has no influence on GnRH neurons. Since GnRH neurons are suppressed during childhood and activated with the onset of puberty, electrophysiological activities of GnRH neurons also may also be different according to the developmental stage. Since we examined the acute effects of rhGH administration on the electrical excitability of GnRH neurons, it is difficult to compare the impact of rhGH on pubertal acceleration in human beings. Further work with large numbers of GnRH neurons and variety concentration of rhGH is required to reconcile our findings and to determine the effect of rhGH on hypothalamic GnRH neurons.
Acknowledgment
This study was supported by Inje University School of Medicine (2009).