Obstructive sleep apnea syndrome in children: Epidemiology, pathophysiology, diagnosis and sequelae
Article information
Abstract
The prevalence of pediatric obstructive sleep apnea syndrome (OSAS) is approximately 3% in children. Adenotonsillar hypertrophy is the most common cause of OSAS in children, and obesity, hypotonic neuromuscular diseases, and craniofacial anomalies are other major risk factors. Snoring is the most common presenting complaint in children with OSAS, but the clinical presentation varies according to age. Agitated sleep with frequent postural changes, excessive sweating, or abnormal sleep positions such as hyperextension of neck or abnormal prone position may suggest a sleep-disordered breathing. Night terror, sleepwalking, and enuresis are frequently associated, during slow-wave sleep, with sleep-disordered breathing. Excessive daytime sleepiness becomes apparent in older children, whereas hyperactivity or inattention is usually predominant in younger children. Morning headache and poor appetite may also be present. As the cortical arousal threshold is higher in children, arousals are not easily developed and their sleep architectures are usually more conserved than those of adults. Untreated OSAS in children may result in various problems such as cognitive deficits, attention deficit/hyperactivity disorder, poor academic achievement, and emotional instability. Mild pulmonary hypertension is not uncommon. Rarely, cardiovascular complications such as cor pulmonale, heart failure, and systemic hypertension may develop in untreated cases. Failure to thrive and delayed development are serious problems in younger children with OSAS. Diagnosis of pediatric OSAS should be based on snoring, relevant history of sleep disruption, findings of any narrow or collapsible portions of upper airway, and confirmed by polysomnography. Early diagnosis of pediatric OSAS is critical to prevent complications with appropriate interventions.
Introduction
Obstructive sleep apnea syndrome (OSAS) is defined as a disorder of breathing during sleep characterized by prolonged partial airway obstruction and/or intermittent complete obstruction (obstructive apnea) that interrupts normal ventilation during sleep and normal sleep patterns1). OSAS is usually considered as an extreme of "sleep-disordered breathing" spectrum encompassing primary snoring, upper airway resistance syndrome, obstructive hypoventilation, and OSAS. Sleep-disordered breathing occurs during sleep and is exacerbated by sleep2). Primary snoring occurs when snoring is not associated with ventilatory abnormalities such as apnea, hypopnea, hypoxia, or hypercapnia3). Upper airway resistance syndrome is characterized by increasing negative intrathoracic pressure during inspiration without apparent apneic or hypopneic events, resulting in increased respiratory arousals leading to increased sleep fragmentation and daytime sleepiness. Obstructive hypoventilation is common in obese children, and diagnosed by snoring, reduced ventilatory drive with hypercapnea without apparent sleep apneas, or respiratory arousals4). Untreated sleep-disordered breathing in children has been reported to be related with various problems such as attention deficit/hyperactivity disorder, poor academic achievement, and behavioral problems. It may even cause more serious morbidities, such as growth failure, cor pulmonale, and systemic hypertension5). Moreover, recent studies suggest that primary snoring may not be as innocuous as previously thought, with learning, neurocognitive, and behavioral deficits being described in children who snore6, 7). This article reviews the prevalence, pathophysiology, and diagnosis of OSAS in children to improve the understanding of pediatric OSAS, and to promote early diagnosis and treatment to prevent serious complications.
Epidemiology
The prevalence of habitual snoring extremely varies, from 3% to 35% of children under 13 years of age, due to the different definition of habitual snoring and epidemiologic methodologies8,9); however, it has been mostly reported in 8-12% of children from 2 to 8 years of age10-13). OSAS has been estimated to affect about 2-3.5% of children8, 13,14). OSAS prevalence has 2 peak periods. The first peak occurs in children from 2 to 8 years of age, with the presence of enlarged adenoid and/or tonsils. A second peak arises during adolescence in relation with weight gain. Although gender differences have not been observed in the prevalence of OSAS among prepubertal children, some adolescent boys has been more affected than girls in a few reports12). In adults, most studies have estimated a sex-specific prevalence of a 2- to 3-fold greater risk for men than women15). In an epidemiologic study for Korean adolescents from 15 to 18 years of age, the prevalence of snoring and OSAS were 11.2% and 0.9%, respectively. Snoring was more common in boys (12.4% in boys vs. 8.5% in girls)11). The prevalence of sleep-disordered breathing in Korean children has not been reported.
Pathophysiology of Childhood OSAS
The upper airway consists of the nose, pharynx, larynx, and extrathoracic trachea, which have important physiologic functions such as respiration, swallowing, speech and vibration, and local immunity. The pharynx is a collapsible segment that performs these functions under the balanced control of dilator and constrictor muscles. During the waking state, upper airway collapse is prevented by keeping an increased pharyngeal neuromuscular tone16). This mechanism, however, is attenuated during sleep, predisposing the upper airway to collapse17).
The main risk factors for OSAS in adults are obesity and male sex, which are related to the propensity for repetitive upper airway collapse18). In younger children, the major risk factor for the development of OSAS is adenotonsillar hypertrophy19). Adenoid and tonsils grow progressively during childhood20-22), whereas the skeletal boundaries of the upper airway slowly expand. Between the ages of 3 and 8 years, the tonsils and adenoid are largest in relation to the underlying upper airway size, which makes a narrow upper airway. This size disparity coincides with the peak incidence of pediatric OSAS5). Neuromuscular factors for upper airway patency are affected by central ventilatory drive, arousal responses to respiratory occlusion (arousal threshold), and upper airway reflexes23, 24). It is suggested that central ventilatory drive is increased during childhood and then declines gradually with age25, 26). This increased central ventilatory drive during sleep accounts for the increased upper airway reflexes and tone in children, resulting in less collapsibility than in adults. Thus, children have a specific breathing pattern of obstructive hypoventilation rather than discrete, cyclic obstructive pattern that is commonly seen in adult sleep-disordered breathing27). Children also have a higher arousal threshold than adults; the younger the child, the higher the threshold of arousal28). Children are, therefore, less likely to arouse in response to upper airway obstruction than adults, accounting for the preservation of sleep architecture29).
In obese children, excessive deposition of fat tissue within the muscles and tissue surrounding the upper airway leads to reduced airway size and increased airway resistance22). Reduced lung volumes and decreased central ventilatory drive in obese children also contribute to compromised upper airway patency30). Nowadays, obesity is a major cause of pediatric OSAS in the western countries due to the dramatic increase of the prevalence of obesity in children. A large epidemiologic study showed that obesity is the most significant risk factor for developing OSAS in children between 2 and 18 years of age, with an odds ratio of 4.531). Recently, it is suggested that pediatric OSAS with obesity would be classified as "pediatric OSAS type 2" since it has similar clinical features with adult OSAS32).
Nasal mucosal edema induced by allergic rhinitis, which increase nasal resistance, may also exacerbate or induce sleep-breathing disorder in children and adolescents1). Craniofacial anomalies such as midfacial hypoplasia, small nasopharynx, and/or micrognathia in Pierre Robin sequence, Apert syndrome, and Marfan syndrome are also important risk factors for developing OSAS19). OSAS presents in 30% to 60% of individuals with Down syndrome, related with decreased nasopharyngeal surface area, ventilatory volume, and hypotonia19, 33, 34). Hypothyroidism, a frequent complication of Down syndrome, is another risk factor, which leads to hypotonia. Neuromuscular disease such as progressive muscular dystrophy is related with reduced upper airway muscle tone, resulting in upper airway collapse.
Genetic Risk Factors
Genetic risk factors have been identified in the development of OSAS35). Asian adults tend to have more severe OSAS for an even lesser degree of obesity than Caucasians36). The short skull base has been noted as an ethnic risk factor in Asians. Asian children also have more severe OSAS than Caucasians, although prevalence is relatively lower37). In African Americans, thick soft tissue dimensions such as tongue mass and oral mucosa are risk factors for OSAS. African American children have been shown to have 4- to 6-fold higher risks than white children, independent of other factors such as obesity, premature birth, and maternal smoking38).
Clinical Manifestations
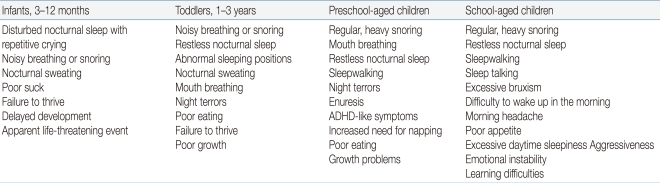
A wide range of symptoms and signs are associated with OSAS in children depending on their developmental stages (Table 1)39). Snoring is the most common presenting complaint of children and adolescents with OSAS. Parents may describe chest wall retractions, paradoxical breathing, and sometimes pauses in breathing. Restless sleep with frequent changes of body position is also well described among children with OSAS, although many parents consider it a normal behavior during sleep in childhood. Excessive sweating during sleep may be related with labored breathing. Abnormal sleep positions such as hyperextension of the neck in infants and abnormal prone position may indicate the possibility of having sleep-disordered breathing. Night terror and sleepwalking are frequently accompanied by sleep-disordered breathing during slow-wave sleep in children and adolescents with a positive family history of parasomnias40). Children with OSAS are high risk for enuresis, which may resolve when the OSAS is adequately treated41). Excessive daytime sleepiness is a typical symptom in adolescents with OSAS, whereas hyperactivity or inattention is predominant in preadolescent children with sleep-disordered breathing. Morning headaches and poor appetite may present in OSAS, which may be due to carbon dioxide retention, sleep fragmentation, or gastroesophageal reflux.
Sequelae of OSAS
Untreated pediatric OSAS can result in serious morbidity in neurobehavioral, cardiovascular, and somatic growth and development. Many studies have shown evidence for clear associations between OSAS and hyperactivity, attention deficit, and other behavioral problems such as social withdrawal or aggression42-45). The prevalence of attention deficit/hyperactivity (ADHD) in the school-age population is 8-10%46), while 20-30% of children with snoring and/or OSAS have clinically significant problems with inattention and hyperactivity47). This ADHD-like features in children with OSAS may result from repeated sleep disruptions and intermittent hypoxic episodes that affect prefrontal executive function such as working memory, behavioral control, analysis, organization, and self-regulation of motivation42, 48). The prefrontal cortex is also believed to be responsible for the regulation of arousal, sleep, affect, and attention, as well as executive functions49). Behavioral deficit and executive dysfunction in children with OSAS have been also shown to have negative impact on learning and school performance50, 51). A number of studies have suggested that therapeutic intervention of OSAS such as adenoidectomy and/or tonsillectomy have a significant improvement of not only the abnormal behaviors such as hyperactivity, inattention, and aggression but also cognition and school performance47, 52-55).
Cor pulmonale with heart failure and pulmonary hypertension were not an uncommon mode of presentation in children with a history of OSAS56, 57); however, they are now rare due to early detection and treatment of OSAS. Although obvious right heart failure now occurs less often, asymptomatic pulmonary hypertension may be common58). Systemic hypertension can occur57). The pathophysiology of cardiovascular complications is as follows: intermittent upper airway obstruction during sleep in OSAS patients that have induced an exaggeration of continuous negative intrathoracic pressure swings, leading to a second series of sustained alterations of blood pressure and endothelial function, and eventually changes in cardiac structure and function occurs probably via oxidative stress and increased sympathetic tone59-65). Increased proinflammatory cytokine such as interleukin-6 or C-reactive protein have been noted in children with OSAS, which may be linked to endothelial dysfunction and atherogensis66). It is suggested that systemic inflammation is a consequence of OSAS even in the absence of obesity, and is reversible after treatment of OSAS in most patients67). Failure to thrive is not a common consequence of OSAS in children; however, growth spurt after adenotonsillectomy is commonly reported68, 69). Growth failure is possibly related to a combination of anorexia and decreased oral intake, increased energy consumption from increased work of breathing, and alternations in nocturnal growth hormone secretion patterns70, 71). Many studies have been shown that catch-up growth is related with increased secretion of insulin-like growth factor-1 (IGF-1) and IGF-binding protein 3 (IGFBP-3) after adenotonsillectomy, both of which are highly correlated with diurnal growth hormone secretion and reflect mean daily growth hormone levels70, 72-74).
Diagnosis
Diagnosis of OSAS in children is made on the basis of sleep history, physical examination, and polysomnographic findings (Table 2)75). OSAS should be suspected on parental complaints about their children's sleep. Frequent napping or excessive sleepiness in the classroom is a major clue to sleep problems in older children. Clinicians can use a sleep log, sleep diary, or sleep questionnaire to efficiently identify the traits of a patient's sleep. Although a thorough history of sleep is very important in the diagnosis of sleep disorders, studies have shown that OSAS cannot be differentiated from primary snoring by history alone1). A comprehensive physical examination of the upper airway from the nose to the oropharynx can help find any anatomical narrowing; that is, a deviated nasal septum, enlarged inferior turbinate, overcrowding of teeth, enlargement of adenoid with/without tonsillar hypertrophy, or the presence of a high and narrow hard palate. Mallampati score is helpful in evaluating the patency of airway related to tonsil size, particularly for older and obese children76) (Fig. 1). An adenoid face with mouth breathing at the waking state is an important clue in detecting sleep-disordered breathing. Inspection of the lateral facial profile is helpful to evaluate for retrognathia, micrognathia, or midfacial hypoplasia.
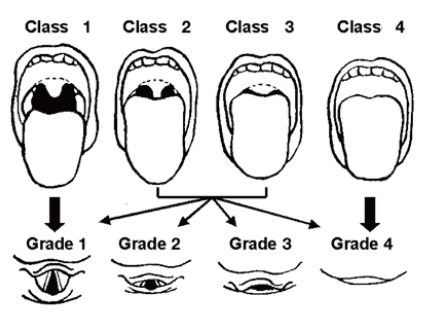
Mallampati score. Class 1: full visibility of tonsils, uvula, and soft palate. Class 2: visibility of hard and soft palate, upper portion of tonsils, and uvula. Class 3: soft and hard palate and base of the uvula are visible. Class 4: only hard palate is visible. Higher scores are correlated with having OSAS.
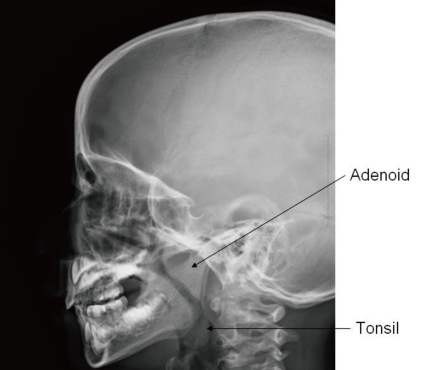
In advanced cases of OSAS, a loud second pulmonary heart sound may manifest as an evidence of pulmonary hypertension63). Assessment for growth and development should not be omitted because growth impairment and delayed development are frequently associated in children with OSAS77). Paranasal sinus with neck lateral view in X-ray is a simple but very useful method for the detection of sinusitis or adenoid hypertrophy (Fig. 2). Endoscopy performed under sedation is useful in localizing the region of maximal airway restriction. This technique is reserved for children with complicated airway structure and altered collapsibility such as congenital craniofacial anomaly78).
Polysomnography
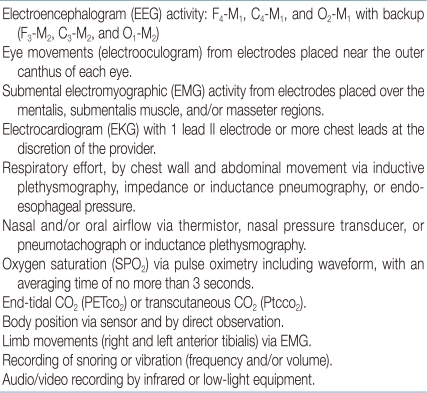
The American Academy of Pediatrics (AAP) suggested a clinical guideline for childhood OSAS in 200256) (Table 3). The AAP recommended polysomnography (PSG) as the only gold standard method for the diagnosis of pediatric OSAS. While they accepted the use of audiovisual taping and pulse oximetry recording as screening studies for OSAS, PSG should be followed in clinically suspected children if these screening tests do not support OSAS. A guideline for PSG in children is recommended by the American Academy of Sleep Medicine (AASM) in 2007 (Table 4)79). Supervision by a trained technician is required throughout the study, with additional record keeping of unusual events or behaviors during the night. An extended full EEG channel is useful for differentiation of nocturnal seizure disorders (e.g., nocturnal frontal lobe epilepsy) from parasomnias such as sleepwalking. Endtidal CO2 measurement can also help assess gas exchange. This is particularly important in children with obesity or neuromuscular diseases, who have a higher risk for hypoventilation78). Balloon esophageal manometry, which can detect upper airway resistance by measuring the intrathoracic negative pressure, is now rarely used in children due to intolerability. New techniques such as pulse transit time or cyclic alternating pattern on EEG are becoming more convenient tools to detect arousals during PSG. These methods are more sensitive and may predict neurocognitive and/or cardiovascular outcomes in children with OSAS80-82).
The PSG parameters that are mostly used in evaluating for OSAS are the apnea-hypopnea index (AHI), apnea index (AI), respiratory event-related arousals (RERAs), and respiratory disturbance index (RDI). AHI indicates the number of apneic and hypopneic events per hour of sleep. Apnea is defined as a complete interruption of airflow lasts at least 2 breath periods in children or 10 seconds in adult, whereas hypopnea is defined as a ≥50% reduction in airflow with an arousal, awakening, or ≥3% desaturation for same durations by using a nasal cannula-pressure transducer79) (Fig. 3). AI indicates the number of obstructive and/or central apneic events per hour of sleep. RERA is defined as a sequence of breath lasts at least 2 breath periods, which does not meet the criteria for apnea or hypopnea but is accompanied by increasing respiratory effort, leading to an arousal from sleep. The AHI and RERA may be reported together as the RDI. The diagnosis of OSAS in children is defined as AHI index >1, according to the criteria of the AASM75), although different criteria for childhood OSAS, such as AI >1 or RDI >1.5, have been suggested in several studies39, 83). Sleep-related hypoventilation may be scored when >25% of the total sleep time is spent with a CO2 >50 mm Hg, measured by end-tidal CO2 (PETco2) and/or transcutaneous CO2 (Ptco2) sensors79, 84) (Table 5). It should be noted that common PSG parameters such as AI, AHI, RDI, and the nadir of oxygen saturation (SpO2) are helpful in evaluating the severity of OSAS, but they may not completely identify sleep-disordered breathing in children since PSG has not been well standardized in its performance or interpretation56). Other important clinical parameters such as paradoxical respiration, duration of abnormal sleep position, and frequency of body position change may be quantified and included as criteria for the diagnosis of childhood OSAS85). Therefore, a thorough validity study for current PSG parameters with the development of new diagnostic criteria for pediatric OSAS is mandatory.
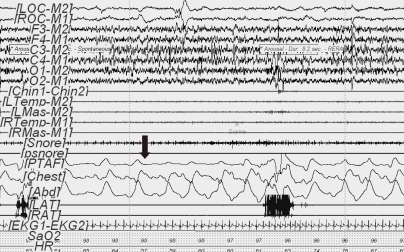
Obstructive hypopnea (60-second PSG epoch) in REM sleep. The event (arrow) was initiated by diminished nasal pressure airflow (TPAF) accompanied by paradoxical respiration leading to arousal. This respiratory event was associated with a ≥50% decrease in the amplitude of the nasal pressure signal. Ocular movement is seen on the electrooculogram (LOC-M2, ROC-M1).
Conclusion
Pediatric OSAS has been commonly overlooked and underdiagnosed by both parents and clinician until now. Clinicians should be familiar with the manifestations of OSAS because children have various clinical symptoms and signs according to their developmental stages. OSAS in children are also different from OSAS in adults, in particular with respect to pathophysiology, gender distribution, and nocturnal and daytime symptoms. Children with OSAS but were not treated may have serious morbidities such as neurobehavioral, cardiovascular, and impairment of growth and development, most of which are reversible by early detection and treatment. Although clinical parameters in the pediatric PSG field are still evolving, PSG is a good diagnostic tool for the diagnosis of pediatric OSAS.