Clinical characteristics of children with 2009 pandemic influenza A (H1N1) admitted in a single institution
Article information
Abstract
Purpose
This study aims to investigate the clinical characteristics of children diagnosed with the novel influenza A (H1N1) in the winter of 2009 at a single medical institution.
Methods
Out of 545 confirmed cases of influenza A (H1N1) in children, using the real time RT-PCR method at Kosin University Gospel Hospital from September to December of 2009, 149 patients and their medical records were reviewed in terms of symptoms, laboratory findings, complications and transmission within a family.
Results
Median age of subjects was 7 years (range: 2 months-18 years). New cases increased rapidly from September to reach a peak in November, then declined rapidly. Most frequently observed symptoms were fever (96.7%), cough (73.2%), rhinorrhea (36.9%) and sore throat (31.5%). Average body temperatures on the 1st, 2nd and 3rd hospital day were 38.75±0.65℃, 38.08±0.87℃ and 37.51±0.76℃, respectively. Complete blood counts and biochemical tests performed on the first admission day showed within the reference values in most cases. Of the 82 patients with simple chest radiography, 18 (22%) had pneumonic lesions; multi-focal bronchopneumonia in eleven, single or multi-segmental lobar pneumonia in five, and diffuse interstitial pneumonia in two patients. All of the 149 patients improved from their symptoms and discharged within 9 days of admission without any late complication.
Conclusion
Children with 2009 pandemic influenza A (H1N1) at our single institution displayed nonspecific symptoms and laboratory findings, resembling those of common viral respiratory illnesses, and did not appear to develop more severe disease.
Introduction
In late March and early April 2009, an outbreak of influenza A (H1N1) infection was detected in Mexico. At first, it was called 'swine influenza' but, subsequently the name was changed to influenza A (H1N1) by the WHO's recommendation. On June 11, 2009, the WHO raised its pandemic alert level to the highest level, phase 6, indicating widespread community transmission throughout at least two continents1). In Korea, as of January 19, 2010, there have been 218 deaths in patients with laboratory-confirmed influenza A (H1N1), since the first case was identified on May 2, 20092).
High rates of infection, morbidity and mortality have been noted among children and young adults across the globe. About 78% of the patients with influenza A (H1N1) were younger than 30 years old3) with the most reported cases in the age range of 14 to 20 years. In Korea, the number of reported cases among students increased rapidly since the middle of October 20094).
The symptoms of influenza A (H1N1) were known not to be different from those of common viral respiratory illnesses or seasonal inf luenza, though the real-time RT-PCR test can differentiate between them. To investigate the clinical characteristics of children of the pandemic influenza in the winter of 2009, we reviewed the medical records of 149 children who had admitted to our institution.
Subjects and methods
1. Subjects
From September to December 2009, in Kosin University Gospel Hospital, 1,037 children under the 19 years of age presented with influenza like illness were tested for 2009 influenza A (H1N1) by real time reverse transcription-polymerase chain reaction (rt RT-PCR) method. Among them, 545 patients (52.6%) were confirmed. Only 149 patients (27.3%) of them were admitted to pediatric ward or cared at emergency room more than 6 hours, and their medical records were available to study symptoms, contact history with a confirmed case, and complications on follow-up. Of the 149 patients, 79 cases underwent laboratory exams including inflammatory indicators and chest radiograms, as they had been cared at pediatric ward more than 2 days.
2. Methods
1) Collection of specimen for diagnosis
Sterile swabs with cotton tips and wooden shafts were inserted into the oral cavity of the patients. The throat swab was rubbed on the tonsillar surface or the pharyngeal mucosa that presented the most secretion under visual inspection. The swab was removed from the mouth and the tip of the swab was carefully inserted into a bottle containing 1 ml of virus transport media.
2) Real time RT-PCR assay
(1) Extraction of RNA and synthesis of cDNA
Respiratory specimens were put into the Abbott m2000sp automatic sample preparation machine (Abbott Molecular Inc., Des Plaines, Illinois, USA) and RNAs were extracted using the Abbott mTM sample preparation system. Viral RNA was reverse transcribed into complementary DNA (cDNA) using a swine-lineage influenza A (H1N1) generic primer. The subsequent PCR amplification and real-time detection of cDNA are performed on Abbott's m2000rt amplification machine.
(2) Used probes and condition for real-time RT-PCR
Each RNA extract sample was tested by separate primer/probe sets: InfA, Universal swine (swFluA), Swine H1 (swH1) and RNaseP (RP). The RNaseP primer and probe set targets the human RNaseP gene and thus served as an internal positive control for human nucleic acid.
3) Criteria of positive reaction
The test for H1N1 influenza A virus is regarded positive if the specimen is positive to either swFluA or swH1 probe, with a concomitant positive reaction to both InfA and RNaseP (RP) probes.
4) Data collection
Medical charts of the patients who were confirmed as novel H1N1 influenza A under the age of 19 were reviewed in terms of demographic features, presenting symptoms, and risk factors for influenza. Fever was defined as a temperature of ≥37.8℃. Body temperature was measured mainly at the forehead or retroauricular area by feverscan (Thermofocus®) and occasionally in the ear by infrared tympanic membrane temperature thermometer (Thermoscan® PRO-LT, Thermoscan, Minnesota, USA). For patients who were admitted, studies of peripheral blood counts, erythrocyte sedimentation rate, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), high sensitivity C-reactive protein (HS-CRP), creatine phosphokinase and simple chest X-ray findings were analyzed. To gain additional information about complication or development of secondary patients within the households, a telephone call was given to each family.
Results
1. Characteristics of the patients
The mean age of the 149 patients was 7.0 (±4.1) years with a range of 2 months to 18 years. Patients are most predominant in the age group of 6-18 years (n=91, 61.1%). Of the 149 patients, 58 were younger than 6 years, and among the seven patients who had bronchial asthma 6 were in the age group of 6-18 years. Accordingly, the proportion of patients considered to belong to a 'high risk group' based on age and underlying medical condition was 43.0% (64/149).
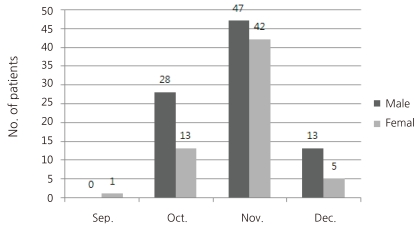
During the 4 months period, there was 1 patient in September, and there were 41 in October 89 in November and 18 in December. So, the numbers of new patients increased rapidly from September to reach a peak in November, then declined rapidly (Fig. 1).
23 (15.4%) of the 149 patients had close contact history with confirmed cases mostly from kindergarten or school (n=13), family (n=7) and their relatives (n=3). The remaining 126 (84.6%) had no known history of contact with confirmed cases.
2. Presenting symptoms and signs of the patients
Of the 149 patients, the most common symptom was fever (n=144, 96.7%). The next common symptoms were cough (n=109, 73.2%), rhinorrhea (n=55, 36.9%), sore throat (n=47, 31.5%), headache (n=26, 17.4%) and sputum (n=21, 14.1%) in descending order of frequency. Other symptoms included were vomiting (n=13, 8.7%), abdominal pain (n=11, 7.4%), dizziness (n=9, 6.1%), dyspnea (n=6, 4.0%), myalgia (n=5, 3.4%), chill (n=4, 2.7%), stuffiness (n=4, 2.7%), diarrhea (n=2, 1.3%), stridor (n=2, 1.3%), nausea (n=1, 0.7%), convulsion (n=1, 0.7%), and skin rashes (n=1, 0.7%) (Fig. 2). On physical examination of the 149 patients, injected tonsillar pharyngeal mucosa were noticed in 20 patients (13.4%), and rales or wheezings were present in 10 patients (6.7%).
3. Hematologic and biochemical laboratory findings
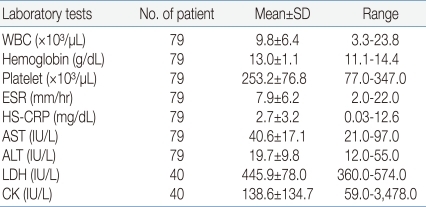
Of the 149 patients, 79 were admitted to pediatric ward, and a complete blood counts were made with peripheral blood. 68 (86.1%) of them showed leukocyte count in the range of 4,000×12,000/µL, while 9 (11.4%) showed under 4,000/µL and 2 (2.5%) showed above 12,000/µL. Patients had thrombocytopenia less than 150,000/µL in 10.1% (8/79). ESR were increased above 15 mm/hr in 17.7% (14/79). Serum levels of HS-CRP showed under the 0.75 mg/dL in 49.4%, between 0.75 to 5.0 mg/dL in 36.7%, above 5.0 mg/dL in 13.9%. No patient showed serum levels of AST and ALT more than 100 IU/L. Serum levels of creatine phosphokinase were checked in 40 patients, and 12 (30%) of them had values more than the upper reference range of 130 U/L (Table 2).
4. Chest radiographic findings of the patients
Chest X-rays were performed in 82 patients at the time of admission or at first visit, Among them, 59 (72.0%) had normal findings, while 18 (22.0%) presented images compatible with pneumonia (Table 3). Those 18 cases were able to classify into three main radiographic patterns; multifocal bronchopneumonia (n=11), single or multisegmental lobar pneumonia (n=5), and diffuse interstitial pneumonia (n=2). Two patients with lobar pneumonia associated with atelectasis and emphysema in one case, and pleural effusion in the other.
5. Clinical course and intra-familial transmissibility
Of the 149 patients, 79 (53.0%) were admitted and 70 (47.0%) were taken care of at emergency room for more than 6 hours. Only one patient with multi-segmental lobar pneumonia associated with atelectasis and emphysema required oxygen inhalation through facemask for a day on the first day of admission. None of the patients required mechanical ventilation or admission to an intensive care unit.
The average length of hospital stay of the patients was 4 days (range of 2-9 days). Body temperatures were available to monitor for 4 consecutive days in 48 patients and 5 days in 30 patients. Average body temperatures on the 1st, 2nd, 3rd, 4th and 5th hospital day were 38.75±0.65℃, 38.08±0.87℃, 37.51±0.76℃, 37.00±0.63℃ and 36.85±0.61℃, respectively, showing rapid defervescence from the 3rd hospital day (Fig. 3). Distribution of documented temperatures of 77 patients upon initial visits were ≥40℃ in 2.6% (2/77), 39.0-39.9℃ in 40.2% (31/77), 38.0-38.9℃ in 44.2% (34/77) and < 38.0℃ in 13.0% (10/77).
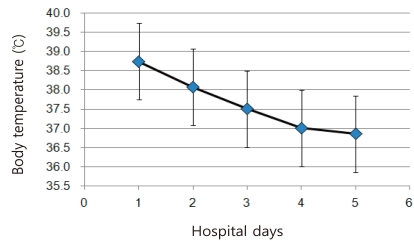
Changes in body temperature (average±standard deviation) during the first 4 hospital days in 48 patients and in the 5th day in 30 patients.
Most of the pneumonic lesions observed on chest radiographs of the 18 cases were undetectable within 9 days of admission. All of the 149 patients had no remaining symptoms or complications on follow up visits at OPD or according to telephone surveys.
As two families had concurrent infections in their brothers, the rate of concurrent infection among the total 147 families was 1.4% (2/147). In sixteen families, twenty infected patients developed secondarily within the households. These 20 new patients were comprised of younger siblings (n=7, 35%), mothers (n=6, 30%), elder siblings (n=4, 20%) and fathers (n=3, 15%). The mean generation time or serial interval time (time from the onset of symptoms in the primary case until the onset of symptoms in a secondary case) was 3.4 (±0.7) days. Most of these data were collected by follow-up telephone interviews with their parents.
Discussion
Annual morbidity due to influenza is highest in children, in whom the attack rates are often 30-50%5). Recent studies have documented that infants and young children without underlying medical conditions are hospitalized for influenza-attributable illnesses at rates of 6-15%6). Persons under the age of 30 years had little evidence of cross-reactive antibodies to the pandemic Influenza A (H1N1), whereas 39 of 115 persons (34%) born before 1950 had preexisting cross-reactive antibodies7). Serologic studies of persons who received vaccination with seasonal influenza vaccines suggest that seasonal influenza vaccines will not provide protection against novel influenza A (H1N1) virus. Among adults, cross-reactive antibodies to novel influenza A (H1N1) virus at titers that correlate with protection from illness in studies of seasonal influenza vaccine was detected in 6-9% of those aged 18-64 years and in 33% of those aged >60 years8).
The above-mentioned facts might be the reason why novel influenza A (H1N1) is more common in young ages. The median and average age released from the study of people less than 18 years by the Korean CDC on October 29, 2009 was 7.5 and 8.9 years, respectively2). In our institution, both of the median and average age of the patients were 7.0 years.
The clinical diagnostic criteria may have similar diagnostic accuracy to that provided by the current rapid diagnostic tests. The criteria for the clinical diagnosis of influenza consists of a fever greater than 37.8℃ plus two or more URI symptoms (cough, sore throat, nasal symptoms, myalgia, headache, malaise)9). Among our 149 patients, 77 (51.6%) were compatible with these criteria.
Several rapid antigen and immunofluorescent antibody tests are available for the diagnosis of influenza virus infection. The sensitivity of these tests varies widely, and although some assays are able to distinguish between influenza A and B viruses, they are not able to distinguish between pandemic and seasonal strains of H1N1 influenza A. However, the specificity of these rapid antigen tests seems to be much higher compared to its sensitivity. In our survey, all positive cases for influenza A with rapid antigen test showed positive to rt RT-PCR tests, though the details are not presented here. Confirmation can be made only by rt RT-PCR or culture. Viral culture is too slow to help guide management, and a negative viral culture does not exclude pandemic H1N1 influenza A infection.
The most common clinical symptoms surveyed in all age groups by Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team in USA were fever (94%), cough (92%) and sore throat (66%). In addition, 25% of patients had diarrhea and 25% had vomiting10). The two most common symptoms of fever (96.7%) and cough (73.2%) in our patients was not different from the WHO and CDC reports. However, the low frequencies of gastrointestinal symptoms and general symptoms such as chill, myalgia and malaise in our subjects need further investigation as to whether these symptoms are truly rare in children or may not be reported by young children and improperly recorded by physicians in a busy outpatient or emergency environment.
The Center for Disease Control in Korea surveyed 2,417 patients with 2009 H1N1 influenza A from May to August 19, 2009. The most common clinical findings of the patients were cough (78.1%), fever (77.2%), sore throat (51.12%), rhinorrhea (46.0%), headache (37.4%), myalgia (23.6%), sputum (15.8%), nausea (9.8%), diarrhea (9.3%) and vomiting (5.3%). The average body temperature of the 1,842 patients who had documented temperature upon initial visit was 38.1(±0.9)℃11). Average body temperatures on the 1st, 2nd and 3rd hospital days in our cases were 38.8±0.7℃, 38.1±0.9℃ and 37.5±0.8℃, respectively. On a review of body temperatures of our 77 patients 42.9% (33/77) had temperatures equal to or higher than 39.0℃.
Laboratory tests including complete blood counts and biochemical tests performed at the time of admission were found to fall within the reference values in most of our cases. The serum AST, ALT values were elevated very minimally in a few. One noticeable feature is that 6 of the 40 patients (15%) have values of serum creatine phosphokinase higher than 512 IU/L which could be attributed to accompanying rhabdomyositis, however, none of those patients was documented to have myalgia. One report from Mexico showed 10 of the 16 patients had increased creatine kinase levels (62%) and 5 of them had very high values ranging from 1,099 to 5,122 IU/L12).
Complications of the 2009 pandemic influenza A (H1N1) were similar to seasonal Influenza A (H1N1). Children younger than 2 years old, adults 65 years of age or older, pregnant women and women up to 2 weeks postpartum and persons with certain medical conditions are regarded as high risk groups for severe complications13). Some of these patients experienced a sudden and very rapid deterioration in their clinical condition, usually on day 5 or 6 following the onset of symptoms14). Clinical deterioration is characterized by primary viral pneumonia, and failure of multiple organs including the heart, kidneys and liver.
The CDC of the United States documented that children less than 6 years of age are at high risk of complications from influenza13). However, observation thereafter showed that children less than 2 years of age had a significantly greater risk of hospitalization14). Children taking long-term aspirin therapy or persons with blood disorders such as sickle cell disease or chronic neurological and neuromuscular disorders, neurodevelopmental condition or obesity also have increased risk of hospitalization or morbidity9, 15, 17). In our study, 64 out of 149 patients (43.0%) were classifiable as belonging to a 'high risk group'. Most of our patients showed rapid defervescence with an oral antiviral agent, oseltamivir (Tamiflu®). In a small proportion of patients, the drug was not given because of mild symptom or discontinued because of vomiting.
The mean time interval between infection of a primary case and one of its secondary cases (the mean generation time) of 2009 pandemic influenza A (H1N1) was known to be 2.5-3.0 days18). After close contact most patients presented symptom in 1-4 days19) with mean incubation of 1.4 days, (95% CI: 1.0-1.8 days)8, 18). The mean generation time within the households in our study was 3.4 (±0.7) days.
Neurological long-term pulmonary complication or fatal course did not occur in our patients, however, the number of patients in our study was not enough to say that the 2009 influenza A virus, H1N1 subtype have without risk. Further study will elucidate the severity or epidemiology of the novel influenza A (H1N1) which is expected to continue to spread beyond pandemic.