A family with Townes-Brocks syndrome with congenital hypothyroidism and a novel mutation of the SALL1 gene
Article information
Abstract
Townes-Brocks syndrome (TBS) is a rare autosomal dominant congenital disorder caused by mutations in the SALL1 gene. Its signs and symptoms overlap with other genetic syndromes, including VACTERL association, Pendred syndrome, Baller-Gerold syndrome, and cat eye syndrome. Structural vertebral abnormalities, hypoplasia of the thumb, and radial bone abnormalities, which are not usually associated with TBS, help in the differential diagnosis of these syndromes. We report the case of a family whose members were diagnosed with TBS with congenital hypothyroidism and had a novel SALL1 gene mutation.
Introduction
Townes-Brocks syndrome (TBS) is a genetic condition that affects several parts of the body, including most commonly imperforate anus, abnormally shaped ears, and hand malformations that mostly affect the thumb. Other possible signs and symptoms of TBS consist of hearing loss, foot malformation, heart defects, genitourinary abnormalities, and rarely eye abnormalities1). Intellectual disability, learning problems, and mental retardation have also been reported in about 10 percent of people with TBS2-3).
TBS is confirmatively diagnosed with a mutation in the SALL1 gene, a part of SALL genes that provide instructions for making proteins in the formation of tissues and organs before birth4). The syndrome is inherited by an autosomal dominant pattern or by a sporadic mutation without familial inheritance. We, herein, report a family whose members were diagnosed of TBS with congenital hypothyroidism and had a novel SALL1 gene mutation.
Case report
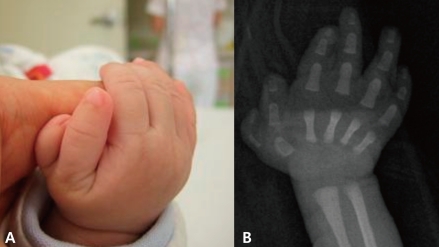
A 4-month-old male infant was born vaginally at 37th week of gestation with a birth weight of 2,600 g after an uneventful pregnancy. At birth, he was noted to have unusually shaped ears with preauricular tags on the right ear (Fig. 1), polydactyly in his right thumb (Fig. 2), overriding toes of both feet (Fig. 3), and imperforate anus (Fig. 4). External ear canals and tympanic membranes were grossly normal. Cardiac echocardiography showed no abnormalities. Abdominal ultrasonography showed hydronephrotic right kidney with a dilated ureter. The imperforate anus was repaired. Analysis on 24-hour urine specimen showed urine output of 1.12 mL/kg/hr; protein 1.75 mg/m2/hr; and the calculated creatinine clearance 14.47 mL/min/1.73 m2/24hr. Temporal bone CT revealed bilateral dysplastic cochleae. Brainstem auditory evoked potential (BAEP) showed bilateral sensorineural hearing loss. Ophthalmologic examination revealed aniridia of the right eye, but orbit computed tomography (CT) was unremarkable. Neonatal thyroid screening test revealed abnormal results (TSH 99.70 µU/mL; T3 102 ng/mL; free T4 1.11 ng/dL), which resulted in thyroxine replacement therapy for congenital hypothyroidism. The thyroid scan showed normal results. Chromosome analysis was normal.
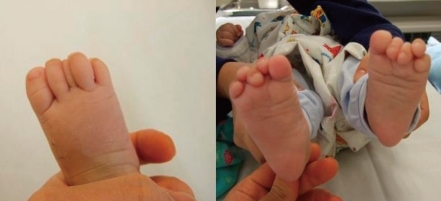
The 2nd and 4th toes overlap the 3rd and 5th toe clinodactyly in the patient with Townes-Brocks syndrome (TBS).
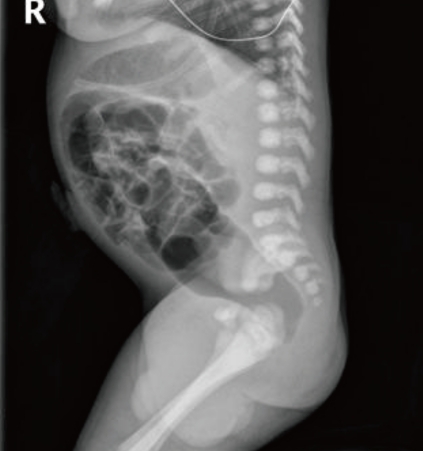
Simple abdomen at birth shows dilation of the descending and rectosigmoid colon, but no gas is seen in the distal rectum.
Family history taking revealed an older brother with imperforate anus, congenital heart defect, malformation of the ears with right sided preauriclar tag, polydactyly of right thumb, and congenital hypothyroidism, who had expired at the age of 3 months from complications of cardiac surgery. The patient also had a 4-year-old sister with bilateral low set ears but no other abnormal features. His mother had a history of a mild anal atresia at birth, bilateral dysplastic ears, hypoparathyroidism, bilateral sensorineural hearing loss which was corrected with hearing aids, and a ventricular septal defect which had spontaneously closed. His maternal grandmother's younger brother had malformation of both ears, but no hearing defect.
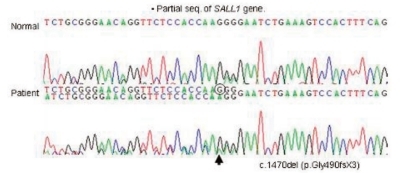
Clinical findings of the patient and pertinent family history suggested the possibility of TBS (Fig. 5). Mutation analysis of exons 1, 2, and 3 of SALL1 gene in the 16q12.1 was performed on the patient and his mother, which showed a heterozygous single base pair deletion at nucleotide position 1470 (c.1470delG) in exon 2 (Fig. 6) which represents a novel SALL1 mutation. This deletion is predicted to cause a frameshift mutation, leading to a truncated SALL1 protein. The parents were against mutation analysis for TBS on their 4-year-old daughter.
Discussion
Townes-Brocks syndrome (TBS) is a rare autosomal dominant multiple malformation syndrome with clinical variability within and between affected families. Characteristic features suggested for TBS include the following: anorectal abnormalities (imperforate anus), abnormalities of the hands (preaxial polydactyly, triphalangeal thumbs), abnormalities of the feet (syndactyly, club foot), deformities of the outer ear ("lop ears") and preauricular tags, and sensorineural hearing loss5). Other reported anomalies include congenital heart defects (VSD, TOF)6, 7), genitourinary abnormalities (impaired renal function or renal failure, hypoplastic or multicystic kidney, posterior urethral valve, vesicoureteral reflux), hypothyroidism, meatal stenosis8, 9), and rarely eye abnormalities (colobomata, aniridia) may be associated. Due to the involvement of multiple organ systems, early recognition of the disorder, appropriate medical management, and genetic counselling of the syndrome is critical. To the best of our knowledge, five cases of TBS have thus been reported to be associated with hypothyroidism5, 10-13). Although thyroid function test is included in the routine neonatal screening test, the result of the test should be reconfirmed in TBS patients.
As features of TBS frequently overlap those seen in other genetic syndromes, TBS may considerably have been misdiagnosed as other genetic disorders. TBS patients may have hand malformation or radial bone abnormalities, but no structural vertebral abnormalities or tracheo-esophageal fistula. VACTERL association may have vertebral abnormalities and tracheo-esophageal fistula, but no ear anomalies or deafness14-16). Baller-Gerold syndrome usually presents with craniosynostosis and thumb anomalies but no hypoplastic thumb17). Cat eye syndrome has tracheo-esophageal fistula and vertebral anomalies but no thumb anomalies14).
TBS is caused by a dominantly inherited defect in the gene encoding the SALL1 putative transcription factor, a protein possibly required for urological, renal, limb, ear, brain, and liver development17). SALL1 gene is located on 16q12.1 and comprises three exons encoding a zinc finger protein thought to act as a transcriptional repressor. Mutations in SALL1 result in prematurely terminated SALL1 protein with loss of all double zinc-finger domains presumably essential for gene function. More than 56 mutations in the SALL1 gene have been identified in patients with Townes-Brocks syndrome. SALL1 mutations have been found in 64.3-83.3% of patients with typical signs of TBS, the majority of which are frame-shift mutations5).
The patient with Townes-Brocks syndrome associated with congenital hypothyroidism in this report was confirmed to have a novel single base pair deletion at nucleotide position 1470 (c.1470delG) in exon 2 of SALL1 gene, resulting in a frameshift mutation which was inherited from his mother.
Acknowledgment
Mutation analysis was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare and Family Affairs, Korea (Grant No. A080588).