Clinical manifestations of CNS infections caused by enterovirus type 71
Article information
Abstract
Purpose
Enterovirus 71, one of the enteroviruses that are responsible for both hand-foot-and-mouth disease and herpangina, can cause neural injury. During periods of endemic spread of hand-foot-andmouth disease caused by enterovirus 71, CNS infections are also frequently diagnosed and may lead to increased complications from neural injury, as well as death. We present the results of our epidemiologic research on the clinical manifestations of children with CNS infections caused by enterovirus 71.
Methods
The study group consisted of 42 patients admitted for CNS infection by enterovirus 71 between April 2009 and October 2009 at the Department of Pediatrics of 5 major hospitals affiliated with the Catholic University of Korea. We retrospectively reviewed initial symptoms and laboratory findings on admission, the specimen from which enterovirus 71 was isolated, fever duration, admission period, treatment and progress, and complications. We compared aseptic meningitis patients with encephalitis patients.
Results
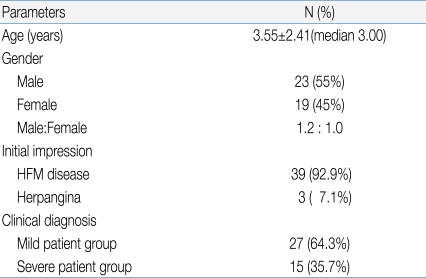
Of the 42 patients (23 men, 19 women), hand-foot-and-mouth disease was most prevalent (n=39), followed by herpangina (n=3), upon initial clinical diagnosis. Among the 42 patients, 15 (35.7%) were classified as severe, while 27 (64.3%) were classified as mild. Factors such as age, fever duration, presence of seizure, and use of intravenous immunoglobulin (IVIG) were statistically different between the 2 groups.
Conclusion
Our results indicate that patients with severe infection caused by enterovirus 71 tended to be less than 3 years old, presented with at least 3 days of fever as well as seizure activity, and received IVIG treatment.
Introduction
Microbiologically, enteroviruses are a genus of RNA viruses of the Picornaviridae family. The genus of enterovirus includes 3 types of poliovirus (PV 1-3), 23 types of coxsackievirus A (CVA 1-22 and 24), 6 types of coxsackievirus B (CVB 1-6), 28 types of echovirus (ECV 1-7, 9, 11-21, 24-27, and 29-33), as well as other types of enterovirus (EV 68-73)1-3).
Enterovirus is spreads through the fecal-oral route. Gastrointestinal infection is followed by primary proliferation in the pharynx or lymph nodes of the small intestine, with spread into various organs in the body4). Clinical symptoms include commonly observed irritation, fever, mild pharyngitis and respiratory symptoms with systemic symptoms such as headache, vomiting and sometimes diarrhea. Most of these symptoms disappear without any complications. However, hand-foot-and-mouth disease (HFMD) caused by EV 71 shows more severe clinical courses. Prominent neurologic complications include meningoencephalomyelitis, poliomyelitis-like paralytic disease, opsoclonus-myoclonus syndrome, benign intracranial hypertension, and brainstem encephalitis. Some cases are mild and reversible but others show rapid progression to neurogenic pulmonary edema, pulmonary hemorrhage, and shock-induced sudden death with a higher frequency in young children in particular5). Since the late 1990s, severe HFMD cases by EV 71 with subsequent mortality have been continuously reported in the West Pacific region including Malaysia, Taiwan, Vietnam and China6-9). In Korea, since the initial report that EV 71 was detected in the fatal case of a 12 month old infant with severe HFMD with neurological complications in May 200910), severe HFMD cases with neurological complications have been consistently observed. Here, we conducted an epidemiologic research of clinical manifestations of young children with central nervous system (CNS) infection by EV 71 to help in the understanding of severe HFMD with neurological complications.
Materials and methods
Among the patients who visited 5 medical centers of College of the Medicine, the Catholic University of Korea (Seoul St. Mary's Hospital, St. Vincent's Hospital, Incheon St. Mary's Hospital, Bucheon St. Mary's Hospital, and Uijeongbu St. Mary's Hospital) from April 2009 to October 2009, 42 patients who were hospitalized and treated for clinically severe HFMD or herpangina with CNS infection were enrolled in this study. CNS infection was confirmed when the patient showed abnormal findings in the CSF study, or in radiologic studies such as CT or MRI. Clinical specimens, stools, nasopharyngeal swab, and cerebrospinal fluid were collected from the patients and sent to the Division of Enteric and Hepatitis Viruses, Korea Centers of Disease Control and Prevention. EV71 was detected by real time PCR. Medical records including initial symptoms and findings at the time of hospitalization, fever duration, hospitalization duration, treatment and complications were retrospectively analyzed. In addition, 42 subjects were classified into two groups based on the severity at presentation: mild patients group and severe patients group. The mild patient group included the patients who showed pleocytosis in CSF study, who showed normal findings in brain CT or MRI, and who recovered without any complications. The severe patient group included the patients who showed either, pleocytosis, abnormal findings in brain CT or MRI, or severe CNS complications like acute flaccid paralysis, ataxia, tremor, motor weakness and coma. The two groups were compared for initial clinical manifestations, gender, age, febrile period, maximal body temperature, presence of seizure, hospitalization duration, test results and immunoglobulin treatment in order to identify prognostic factors for severe CNS complications. Statistical analysis was conducted by using SPSS, version 12.0. Statistical significance was determined as P<0.05 in t-test and chi-square test.
Results
1. Demographic characteristics and clinical manifestations
Among 42 pediatric patients with CNS infection by EV 71, 23 patients were male and 19 patients were female (gender ratio 1.2:1) with a mean age of 3.55 (Table 1).
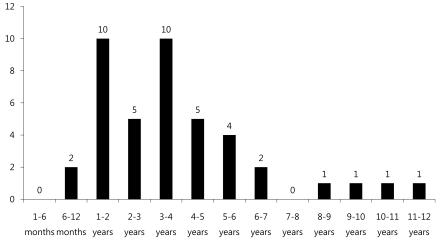
Age distribution of subjects (range: 6 months-12 years old) was 10 patients (24%) for each 1-2 years and 3-4 years old age group, followed by 5 patients (12%) for 4-5 years old age group, 4 patients (9.5%) for 5-6 years old, and 2 patients (4.8%) for 6-7 years old, suggesting decreased disease prevalence with increased age (Fig. 1).
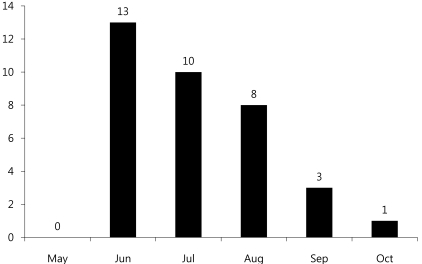
Monthly incidence showed 13 patients diagnosed in June, 10 patients in July, and 8 patients in August, showing significantly higher incidence in summer (Fig. 2).
Among 42 subjects, HFMD was the first clinical diagnosis for 39 patients (92.9%), while herpangina was diagnosed in 3 patients (7.1%).
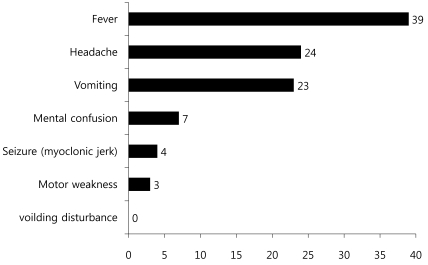
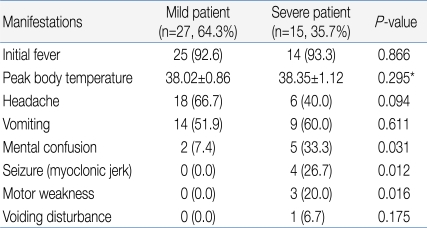
Among 42 subjects, 27 patients (64.3%) were classified into the mild patient group, and 15 patients (35.7%) were classified into the severe patients group (Table 1). For initial clinical symptoms, fever (39 patients; 92.9%) was most frequent, followed by headache (24; 57.1%), vomiting (23; 54.8%), and decreased consciousness (7; 16.7%). Other symptoms included seizure (4; 9.5%), motor weakness (3; 7.1%), and dysuria (1; 2.4%) (Fig. 3).
2. Clinical comparison between mild patient group and severe patient group
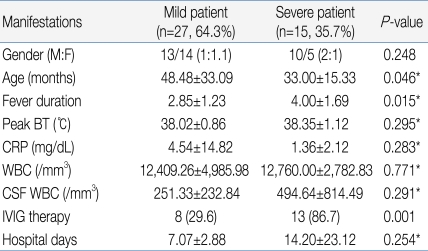
Among the 15 in the severe patient group, decreased consciousness, seizure, and motor weakness were observed in 5, 4, and 3 patients, respectively, as initial clinical symptoms. However, these symptoms were observed in 2, 0, and 0 patients, respectively, out of the 27 patients in the mild group, indicating a significant difference between the two groups with regards to these symptoms (Table 2). The severe patient group showed 33.00 months of mean age, 4.00 days of febrile period, and 38.35℃ of maximum body temperature, while the mild patients group showed 48.48 months of mean age, 2.85 days of febrile period, and 38.02℃ of maximum body temperature, showing a statistically significant difference in mean age and febrile period between the two groups except maximum body temperature (P<0.05) (Table 3).
When laboratory test results were compared, the severe patient group showed an average CRP level of 1.36 mg/dL, peripheral blood white blood cell (WBC) count of 12,760/mm3, and CSF WBC count of 494.64/mm3, while the mild patient group presented with an average CRP level of 4.54 mg/dL, peripheral blood WBC count of 12,409.26/mm3, and CSF WBC count of 251.33/mm3, indicating no statistically significant difference between the two groups (Table 3).
With regards to treatment, 8 of 27 patients in the mild group (29.6%) received IVIG treatment compared to 13 of 15 patients in the severe group (86.7%), showing statistically significant difference between two groups (Table 3). Patients in the mild group were discharged from the hospital fully recovered, regardless of IVIG treatment. In contrast, patients in the severe group were discharged in a neurologically complicated state.
3. Enterovirus 71 detection
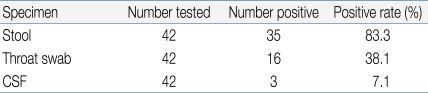
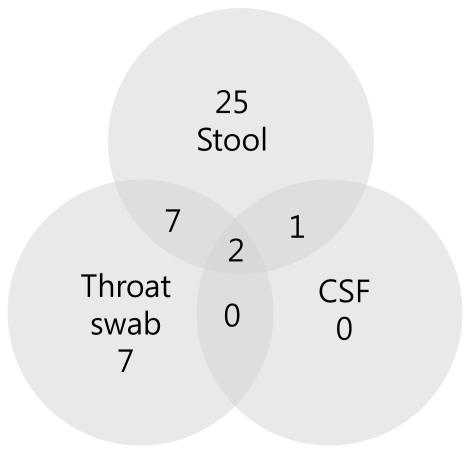
Positive findings were observed in 35 stool specimens (83.3%), 16 nasopharyngeal swab specimens (38.1%), and 3 CSF specimens (7.1%) out of 42 patients (Table 4). In addition, 7 patients showed concomitant positive findings in both stool and nasopharyngeal swab specimens, 1 patient was found to be positive in both stool and CSF specimens, while 2 patients showed positive results in all stool, nasopharyngeal swab, and CSF specimens (Fig. 4).
Discussion
Enterovirus infection is prevalent worldwide, and the infection usually spreads in the warm season, especially summer and autumn, when CNS infection by enterovirus increases5). Based on cases of CNS infection by enterovirus in Korea, the infection first occurs in March; the incidence rate reaches its peak in July and August, and then decreases11-15). In our study, the monthly incidence rate of CNS infection by enterovirus 71 was higher during May to August, and then decreased from September (Fig. 2). In most studies, a gender-specific difference in incidence rate was observed with higher prevalence in males11-15). Consistent with this observation, the gender ratio was 1.2:1 in this study, suggesting a slightly higher prevalence in males (Table 1). It has been reported that most patients were 5 years old or younger12), but there are also reports that the highest incidence rate was observed in the 4-9 years age group13) or 5-10 years of age16). This study showed a broad spectrum of age distribution, ranging from 6 months to 12 years of age, but 60% of patients were 5 years old or younger (1-2 years and 3-4 years age group: 24%, 4-5 years: 12%) with a mean age of 3.55±2.41, showing that most cases are observed in children 5 years old or younger (Table 1).
Most clinical symptoms are asymptomatic or mild, usually with non-specific symptoms such as fever, irritation, agitation, sore throat, headache, myalgia, vomiting, mild abdominal discomfort, and diarrhea5). The initial clinical symptoms in this study also included fever (92.9%), headache (57.1%), and vomiting (54.8%). Since the late 1990s, HFMD by EV 71 has been reported in various West Pacific countries such as Taiwan, China and Vietnam, resulting in many severely infected pediatric patients with CNS complications. These patients showed myoclonic seizure, motor weakness, and neurologic manifestations such as tremor, nystagmus, cranial nerve palsy and seizure, which are highly indicatives of brainstem encephalitis16). Some of these patients showed pulmonary edema or pulmonary hemorrhage with sudden death due to cardiopulmonary collapse or refractory shock16). Consistently, decreased consciousness, seizure and motor weakness as initial clinical symptoms were observed in 5, 4 and 3 patients, respectively, out of 15 patients with severe CNS complications in contrast to 2, 0 and 0 patients, respectively, in the mild patient group, suggesting a significant difference (Table 3). However, no patient in the severe patient group showed pulmonary edema or pulmonary hemorrhage in this study. The risk factors associated with severe CNS complications by EV 71 include a febrile period of 3 days or longer febrile period, 39℃ or higher fever, and increased peripheral blood WBC counts17). In this study, the severe patient group with major CNS complications presented at a mean age of 33.0 months and 4 days of febrile period, while the mild patient group were diagnosed at a mean age of 48.5 months and 3 days of febrile period. This significant difference suggests that patients 3 years of age or younger with 4 days of febrile period or longer may show severe symptoms resulting in major neurological complications. However, no statistically significant difference was observed with regards to maximum body temperature, peripheral blood WBC count, CRP level, and CSF WBC count, indicating that these parameters may have less significant value as prognostic predictors (Table 3).
The standard protocol for enterovirus diagnosis is based on virus culture, but it takes several weeks for confirmation of final results with about 50-70% of sensitivity. CSF specimens are usually used for the analysis of concurrent CNS complication cases, but simultaneous analysis with stool and nasopharyngeal swab specimens can increase the sensitivity5). For nasopharyngeal swab specimens, the specimens should be obtained from nasopharyngeal lymphoid tissues or mucosal epithelia at an early stage of disease course in order to purify viruses. As for stool specimens, viruses can be excreted into stools for several weeks, but a higher success rate of virus culture is obtained when the specimens are collected within 2 weeks of disease onset18, 19). Generally, the detection rate of enterovirus is highest in stool specimens, followed by nasopharyngeal swab specimens, CSF specimens, and blood specimens20). In some cases, however, disease progression is rapid, emphasizing the need for faster methods of diagnosis21). The detection rate of enterovirus in this study was highest in stool specimens, followed by nasopharyngeal swab specimens, and CSF specimens. In contrast to other studies, the detection rate in CSF specimens was low in this study, which may be due to delayed CSF study and consequent delayed transportation of CSF specimens, prolonged storage of specimens prior to transportation, or problems during specimen transportation.
The administration of high dose intravenous immunoglobulin (IVIG) is recommended for the treatment of severe enterovirus CNS infection, and prompt IVIG administration based on early diagnosis of severe CNS infection is required for favorable outcomes5, 17, 21). In this study, 13 of 15 patients (86.7%) in the severe patient group received IVIG treatment while 8 of 27 patients in the mild group (29.6%) received IVIG. There was statistically significant difference with regards to IVIG treatment. However, IVIG may be used in mild patient group to prevent rapid progression to severe clinical courses. With increased overseas travel and use of child care facilities, the risks of imported EV 71 infection and spread are extremely high. In particular, child care centers, kindergartens and schools crowded with children are vulnerable to viral transmission. In 2008, an expanded enterovirus infection resulted in the temporary closing of classes in many schools in Singapore22). In 2009, severe EV 71-induced HFMD with neurological complications was reported in Korea. We are continuously at risk of repeated infections, but no nationwide epidemiological study has been conducted, with many unresolved problems pertaining to early diagnosis. Thus, close nationwide epidemiological investigations and clinical studies of severe HFMD remain to be conducted in the future.