Misdiagnosis of fetus-in-fetu as meconium peritonitis
Article information
Abstract
Fetus-in-fetu (FIF) is a rare congenital condition in which a fetiform mass is detected in the host abdomen and also in other sites such as the intracranium, thorax, head, and neck. This condition has been rarely reported in the literature. Herein, we report the case of a fetus presenting with abdominal cystic mass and ascites and prenatally diagnosed as meconium pseudocyst. Explorative laparotomy revealed an irregular fetiform mass in the retroperitoneum within a fluid-filled cyst. The mass contained intestinal tract, liver, pancreas, and finger. Fetal abdominal cystic mass has been identified in a broad spectrum of diseases. However, as in our case, FIF is often overlooked during differential diagnosis. FIF should also be differentiated from other conditions associated with fetal abdominal masses.
Introduction
Fetus in fetu (FIF) was first described by Meckel in the early 19th century, and it was defined as a mass containing a vertebral axis often associated with other organs or limbs around this central axis in 19351). Since then, this disease entity has been rarely reported. Its incidence is estimated to be approximately 1 per 500,000 births2). Hoeffel et al.3) reviewed a large series of 88 cases of FIF.
Twelve cases have been reported in Korea. With advance in prenatal diagnosis techniques, more than half of the cases have been detected prenatally. In this report FIF which was mimicking cystic meconium peritonitis in prenatal ultrasonography was described. Differentiating a FIF from other cause of meconium peritonitis is discussed with review of literature.
Case report
A 31-year-old primi pregnant woman with minimal prenatal care and no history of fertility medications or in vitro fertilization came to our institution at 33 weeks' gestation. There was no family history of twin pregnancies, congenital malformations or prenatal illness. Obstetric sonography at 26 weeks' gestation revealed a single fetus of appropriate size for gestational age, but bowel wall edema and small bowel obstruction were suspected. Sonography at 29 weeks' gestation showed a large amount of amniotic fluid and the aforementioned findings were not changed. Sonography at 33 weeks' gestation which was performed at our institution showed that a markedly dilated bowel and the bowel obstruction were still suspicious. The amount of amniotic fluid was increased, and a 6.8×6.4 cm pseudocyst and a 4.39×3.22 cm calcified heteroechgenic mass were found (Fig. 1). Otherwise, there were no abnormal findings. Our clinical diagnosis was a giant cystic type of meconium obstruction associated with peritonitis induced by perforation and atresia of the small bowel. The weekly ultrasonographic follow-ups showed no significant interval changes.
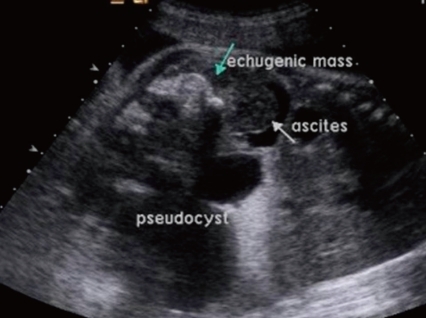
Perinatal ultrasonography findings included ascites, pseudocyst, and echogenic mass which suggested meconium peritonitis.
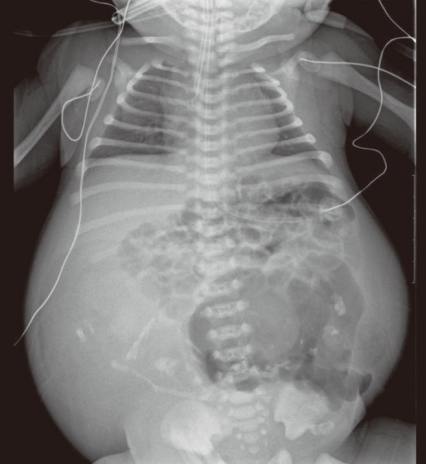
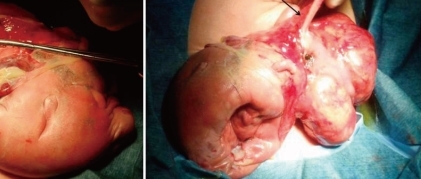
At 38-weeks' gestation, a female newborn weighing 4,120 g was delivered by an elective cesarean section, with Apgar scores of 2, 4 and 9 at 1, 5 and 10 minutes. The baby had a grossly distended abdomen and did not present any evidence of dysmorphic features. The patient had respiratory distress after birth which was probably induced by elevated abdominal pressure due to the abdominal mass and ascites and necessitated assisted ventilation. She was admitted to the neonatal intensive care unit for further evaluation and treatment. Laboratory tests showed no abnormalities except for a slightly decreased albumin level of 3.1 g/dL and an alpha-fetoprotein level of 5,845 ng/mL. Abdominal radiographs showed a large abdominal mass containing multiple calcifications in the lower abdomen (Fig. 2). Abdominal ultrasonography showed a huge solitary and cystic mass which filled most of the abdomen. Abdominal computed tomography (CT) showed a well-demarcated intraabdominal mass measuring 15.6×10.2 cm, with a heterogeneous enhancing solid portion, fatty component and a cystic portion containing multiple calcification or ossification lesions. Both ovaries were not visible. Based on these findings, a teratoma arising from both ovaries was the most likely diagnosis, and she underwent an elective laparotomy at 5 days of age. During surgery, we observed a small amount of ascites with a mass attached to the colon and mesocolon which was thought to be a splenic flexure (Fig. 3). The mass did not have a cystic sac and had a buttock-like lesion with skin and hair follicles, fatty tissue, a finger and a coccyx. A tubular structure was also identified along the mesenteric border of the sigmoid colon, which was suspected of an intestinal duplication cyst. Feeding vessels were not found.
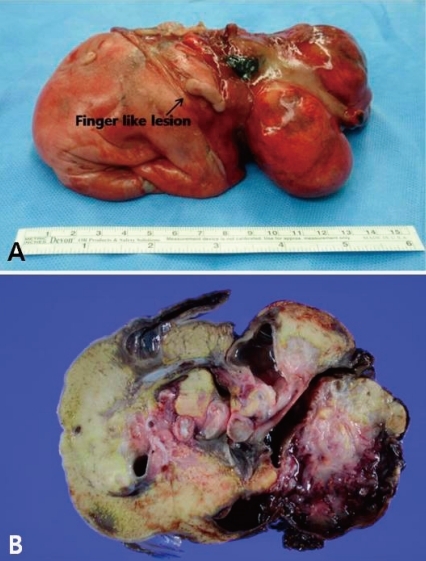
On macroscopic examination, the mass measuring 15×9×5 cm had a smooth surface partially covered with skin or thin membrane and 2 finger-like lesions (Fig. 4). On section, most of the mass consisted of yellowish fat tissue, and the middle portion of the mass had a tubular structure which looked like an intestine. The mass contained a hard cartilage-like lesion and a myxoid gray lesion. Microscopic examination showed a well developed gastrointestinal tract, liver, pancreas and finger as well as 3 germinative layers, but the vertebral axis was not identified.
Discussion
Meckel first coined the term "fetus in fetu" in the late 18th century to describe an encapsulated fetoid tumor within a fetus. After then, Willis1) stated in 1935 that the presence of an axial skeleton distinguishes between FIF and a teratoma, which remains a clinical definition. Gonzalez-Crussi4) defined FIF in a narrower sense as "highly organotypic development and presence of a vertebral axis with arrangement of tissue around this axis". The definition of FIF still remains controversial.
Thirty-one cases of FIF were reported before 1900, and thereafter only 11 cases were reported between 1900 and 19563). Its incidence is very low and is estimated to be 1 per 500,000 birth2). Hoeffel et al.3) reviewed 88 reported cases and their own case. We have reviewed all cases of FIF reported during the 10-year period after 2000. Eighty-one cases were searched. The incidence of FIF has increased and the incidence seems to be higher than in previous reports.
With advances in prenatal diagnosis techniques, more than half of the cases have been detected prenatally. Our case was also detected prenatally, but it was misdiagnosed as cystic meconium peritonitis. Meconium peritonitis represents aseptic peritonitis caused by the spreading of meconium into the fetal peritoneal cavity. Cyst occurs when the site of perforation is not sealed and a thick-wall cyst is formed within the intestinal loops. The cystic type of meconium peritonitis is thought to be a life-threatening condition. Therefore, rapid deterioration of fetal status can be possible in this type. In this regard meconium pseudocyst must be antenatally differentiated from other intra-abdominal cystic images such as urinary bladder distention, ovarian cyst, intestinal duplication, hematoma, teratoma and fetal infection5). FIF can be presented prenatally as an abdominal cystic mass with diffuse calcification or ossification by ultrasonography. Thus, FIF should also be differentiated from meconium pseudocyst. CT or MRI has sometimes been used as a confirmatory diagnostic tool to differentiate between these diseases. The vertebral axis was identified in 91% of FIF cases in the literature3). Definition of FIF still remains controversial. However, if the vertebral axis-like structure is seen within a mass on prenatal imaging, it is also helpful in differential diagnosis.
It is still controversial whether the presence of the vertebral axis should be included in the diagnostic criteria for FIF. Some previously reported cases without the vertebral axis were diagnosed as FIF as in our case. All of these cases had well-organized masses in a craniocaudal direction. Embryology of the FIF has not yet been understood. It is thought to occur at the very early stage of embryo development before the ventral fusion of the lateral body walls. The spectrum is diverse from conjoined symmetric twins to a form of organized teratoma, but FIF is thought to be a different category from teratoma based on its organo-level development, rarity of malignant potential and the possession of the same karyotype with the host. Only a small number of cases underwent genetic studies in the literature. Chromosome analysis was performed on 29 cases, which showed the same chromosomes as the host's. Miura et al.6) reported that in 2 cases undergoing DNA methylation assay, 46.7% and 66.7% of clones from the mass had an unmethylated paternal allele. They suggested that 2 isolated blastocysts originated from one zygote, and one of the two was implanted into the other blastocyst during the process of methylation. In this regard, presence of vertebral axis to diagnosis of FIF may not be a prerequisite factor.
Treatment is complete surgical excision. The excised mass needs pathological examination including genetic testing. Pathological confirmation is essential for a final diagnosis, and genetic testing can provide unambiguous evidence for a fetus in fetu. There have been no reports with a long-term follow-up. Several cases have been reported in older children with a delay in diagnosis and 1 case in a 39-year-old man, was and they showed no malignant potential7).
The prognosis of intracranial FIF is not good. Only 1 case survived, and 2 cases were stillborn, 2 cases were terminated fatally due to intracranial hypertension, and 2 cases were terminated with their parents order. The concurrent presence of a teratoma component is not predictable. Hopkins et al.8) reported a retroperitoneal FIF presenting as a right abdominal mass which proved to be a teratoma with malignant components requiring chemotherapy8). There are 2 other cases with local recurrence as a teratoma without malignant potential in the literature; they were located in the retropenitoneum and recurred at 1 and 2 years of age, respectively9, 10). For this reason, it is necessary to regularly follow up patients with FIF for the surveillance of recurrence.
Conclusion
We report a case of FIF which was misdiagnosed as cystic meconium peritonitis prenatally. Abdominal cystic mass should be differentiated from various conditions. Although FIF is considered as a rare disease, it has been increasingly reported in the literature. Thus, FIF should also be included in the differential diagnosis of cystic masses in fetus. The definition of FIF has not yet been established. Genetic testing can find out its embryologic cause.