Testicular adrenal rest tumors in a patient with untreated congenital adrenal hyperplasia
Article information
Abstract
Testicular adrenal rest tumors (TARTs) are considered to be formed from aberrant adrenal tissue that has become hyperplastic because of elevated adrenocorticotropic hormone (ACTH) in male patients with congenital adrenal hyperplasia (CAH). A 6-year-old boy presented with testicular enlargement and pubic hair. He was diagnosed with CAH complicated by precocious puberty. However, he was not followed-up. At the age of 17, he visited the outpatient clinic because of testicular enlargement and short stature. His right and left testicles were 10×6 cm and 7.5×4.5 cm, respectively. His height was 155.1 cm (standard deviation score [SDS], -2.90). The diagnosis of CAH due to 21 hydroxylase deficiency was confirmed by mutation analysis of CYP21A2. Histological examination of the testes showed large, polygonal, eosinophilic cells with round nuclei and prominent nucleoli, which were suggestive of TARTs. He was treated with dexamethasone for 3 weeks and tumors regressed. Subsequently, dexamethasone was replaced by prednisolone and 9α-fludrocortisone; thereafter, the reduced testis size has been maintained.
Introduction
Congenital adrenal hyperplasia (CAH) is an autosomal recessively inherited disorder of adrenal steroid synthesis, in which glucocorticoid and mineralocorticoid deficiencies lead to a compensatory increase in adrenocorticotropic hormone (ACTH) secretion, which in turn causes adrenocortical hyperplasia and overproduction of intermediate metabolites and adrenal androgens1). Testicular adrenal rest tumors (TARTs) are important complications of CAH, resulting in infertility, and were first described in 19402). TARTs have been observed in 0-94% of CAH patients, dependent on patient selection and the method of tumor detection3). TARTs are thought to arise from aberrant adrenal cells that migrate to the scrotum during the descent of the testes or from pluripotent stem cells in the testes4). The growth of these cells in patients with CAH may be caused by the chronically elevated levels of ACTH or other, as yet unknown, growth promoting factors5). In patients with CAH, poor compliance with treatment increases plasma ACTH levels, accelerating the development of TART. Although TARTs are usually benign, they may obstruct the seminiferous tubule, leading to possible gonadal dysfunction5). Intensive glucocorticoid therapy, which suppresses ACTH secretion, leads to regression of the tumors. If glucocorticoid treatment is ineffective, however, surgical intervention should be considered. We describe here a patient with untreated CAH who presented with huge TARTs, which regressed with dexamethasone therapy.
Case report
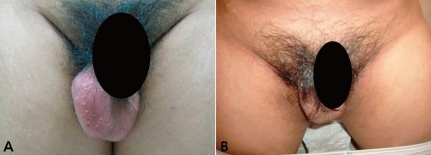
A 2-month old boy was admitted to our primary clinic because of poor weight gain, hyponatremia, and hyperkalemia. Initially, he was thought to have electrolyte abnormalities caused by dehydration and discharged without an exact diagnosis. When he was 6 years old, he manifested voice change, acne, testicular enlargement, and premature pubarche. His height and weight were 124 cm (2.31 SDS) and 27 kg (2.63 SDS), respectively. The volume of each testis was 7 mL, the stretched penile length was 7 cm, and his bone age was 13 years by Greulich and Pyle's method, which was remarkably advanced for his chronological age. His serum 17-hydroxypregnenolone (17-OHP) and testosterone levels were elevated to 480 ng/mL (normal range: 0.32-3 ng/mL) and 2.0 ng/mL (normal range: 0.03-0.1 ng/mL), respectively. He was diagnosed with precocious puberty caused by CAH. He was treated with hydrocortisone (15 mg/m2/day) and 9α-fludrocortisone (0.1 mg/day). However, he did not take medicine after 11 months of treatment. At age 17 years, he visited our outpatient clinic because of bilateral testicular enlargement and short stature. He was scheduled to be treated by surgical resection of testicular tumors because testicular biopsy demonstrated large, polygonal, and eosinophilic cells with round nuclei and prominent nucleoli, which are consistent findings with Leydig cell tumors (Fig. 2). His height and weight were 155.1 cm (-2.90 SDS) and 68 kg (0.49 SDS), respectively, and body mass index (BMI) was 30.2 (>97th percentile). He was normotensive (110/70 mmHg). His right and left testicles measured 10×6 cm, 7.5×4.5 cm, respectively (Fig. 1), with both being of firm consistency. His pubic hair Tanner stage was grade V. Gynecomastia and skin hyperpigmentation were noted. Radiography of left hand and wrist showed a closed epiphyseal plate. His serum 17-OHP concentration was 506.20 ng/mL (normal range: 0.32-3 ng/mL), ACTH level was 187.6 pg/mL (normal range: 2-49 pg/mL), plasma renin activity was 20.0 ng/mL/h (normal range: 0.4-8.8 ng/mL/h), and dehydroepiandrosterone sulfate (DHEA-S) level was 270.6 µg/dL (normal range: 30-555 µg/dL). He had an androstenedione level of 18.0 ng/mL (normal range: 0.57-1.5 ng/mL), a luteinizing hormone (LH) concentration of 0.23 mIU/mL (normal range: 1.54-7.0 mIU/mL), a follicle-stimulating hormone (FSH) level of 1.6 mIU/mL (normal range: 1.54-7.0 mIU/mL) and a testosterone level of 6.13 ng/mL (normal range: 3.5-9.7 ng/mL). His serum sodium and potassium concentrations were 141 mmol/L and 4.1 mmol/L, respectively, and serum α-fetoprotein and β-hCG levels were 1.4 ng/mL (normal range <20 ng/mL) and 1.0 mIU/mL (normal range <5 mIU/mL), respectively. Abdominal computerized tomography revealed a 1.5 cm-sized adrenal adenoma on the left side and focal adrenal hyperplasia on the right side. Mutation analysis of CYP21A2 revealed a compound heterozygote, c.293-13A>G and c.1066C>T (p.R356W). To reduce the size of his tumors and to improve his hormonal profile, he was prescribed ACTH suppressive therapy with dexamethasone (0.25 mg tid). After 3 weeks, the tumors markedly regressed. The volume of each testis estimated using a Prader orchidometer was 25 mL (Fig. 1). His serum 17-OHP and ACTH concentrations had decreased to 1.0 ng/mL and 9.4 pg/mL, respectively, and serum testosterone concentration was reduced to 0.05 ng/mL (normal range: 3.5-9.7 ng/mL). He was switched from dexamethasone to prednisolone (5 mg twice a day) for maintenance, which had no effect on the volume of his testes. Following the switch, 17-OHP concentration was 19.8 ng/mL, ACTH concentration was 45.3 pg/mL, plasma renin activity was 20.0 ng/mL/h, and testosterone level was 3.0 ng/mL.
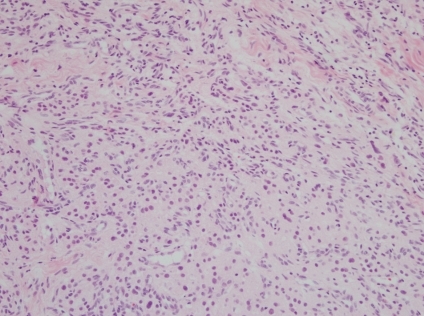
Histopathological examination of a testicular biopsy sample. Large polygonal cells with abundant eosinophilic cytoplasm and separated by fibrous tissue strands were observed. No Reinke crystalloids were observed (Hematoxylin-eosin staining, ×200).
Discussion
TARTs can be the first manifestation of CAH and have been reported in 18% of undiagnosed CAH patients2). TARTs usually develop in untreated or inadequately treated patients with CAH because of chronically elevated ACTH levels. In addition, increased levels of LH during the pubertal period has been found to stimulate tumor growth by binding of LH to receptors located in tumor tissues. Therefore, TARTs are usually detected in adolescence and young adulthood, even in adequately treated patients.
TARTs are usually nodular, firm, well delineated, and separated by fibrous bands. Because of their histopathologic resemblance on microscopy, it is difficult to differentiate TARTs from Leydig cell tumors6). Both tumors contain polygonal cells with abundant eosinophilic or granular cytoplasm in cord-like arrangements separated by dense fibrotic tissue4). TARTs, however, never contain the cytoplasmic rod-like crystalloids termed Reinke crystals, a pathognomonic finding of Leydig cell tumors found in 20-40% of patients with the latter condition7). Several clinical characteristics can be used to differentiate TARTs from Leydig cell tumors. For example, Leydig cell tumors can appear at any age, and only 3% are bilateral8). In contrast, TARTs are more common during adolescence and young adulthood, and are usually bilateral9). Thus, bilateral testicular masses in adolescents should be suspected of having TARTs with CAH although the histologic findings are consistent with Leydig cell tumors. TARTs are benign and reversible without surgical intervention if the tumors are found before stage IV by classification proposed by Claahsen-van der Grinten HL et al.3). No cases of metastatic TARTs have been reported and tumors shrink after high-dose glucocorticoid therapy2). In our patient, TARTs could not be clearly differentiated from Leydig cell tumors histopathologically, but the marked reduction in testicular size following high-dose glucocorticoid therapy suggested that the tumors were TARTs, not Leydig cell tumors. Although TARTs have no malignant features, they can cause severe testicular damage by compressing the seminiferous tubules. Chronic obstruction of the tubules has been found to cause peritubular fibrosis and tubular hyalinization, leading to irreversible testicular damage10). In addition to their mechanical effects, adrenal androgens produced by such tumors may be toxic to Leydig cells and germ cells11). Therefore, early recognition and treatment is required for better prognosis. Because TARTs are located adjacent to the mediastinum testis, only tumors of >2 cm are detectable by palpation. To find small-sized tumors, ultrasonography should be performed in addition to regular digital palpation. Ultrasonography has been found to be as sensitive as magnetic resonance imaging in detecting small TARTs12).
Several treatment modalities have been described that reduce tumor size and improve gonadal function. Because these tumors result from elevated ACTH concentration, intensive glucocorticoid therapy, which suppresses ACTH secretion and leads to regression of tumors, has been found effective. Dexamethasone has a longer half-life than has hydrocortisone (36 to 54 hours vs. 8 to 12 hours) and more effectively suppresses the hypothalamic-pituitary axis13). If the tumors do not respond to dexamethasone, surgical intervention should be considered. Testis-sparing surgery is preferred for prepubertal tumors, with psychological and cosmetic advantages. However, in the case of longstanding tumors in patients with poorly controlled CAH, irreversible testicular damage may persist after surgery10).
Molecular analysis of CYP21A2 in our patient revealed mutations common in CAH of the salt-wasting type, but our patient had not shown any symptoms of adrenal crisis during childhood. This may have been because his TARTs had some features of adrenal cells because of the presence of CYP11B1 and CYP11B2 in tumor tissue14). It is unusual to find a CAH patient of the salt-wasting type who in the absence of treatment, did not present with salt-wasting symptoms. Because TARTs may cause deterioration in semen production as well as testosterone secretion, semen analysis may help to evaluate gonadal function in adults. As poor compliance with medication can aggravate TARTs, patients should be regularly followed-up and controlled with appropriate medication, to prevent residual tumor regrowth.
In conclusion, early detection of TARTs is important in preventing irreversible testicular damage. Patients with CAH should be regularly examined by digital palpation and radiological methods such as ultrasonography, to detect small-sized tumors.
Acknowledgements
This study was supported by a grant (A080588) from the Korean Ministry of Health and Welfare.