Recommendation for the use of newly introduced Tdap vaccine in Korea
Article information
Abstract
Pertussis is an acute respiratory infection characterized by paroxysmal cough and inspiratory whoop for over 2 weeks. The incidence of pertussis has decreased markedly after the introduction of DTwP/DTaP vaccine, but the incidence of pertussis has increased steadily among young infant and among adolescents and adults in many countries. Td vaccine was used in this age group but the increase in pertussis has lead to the development of a Tdap vaccine. The Tdap vaccine is a Td vaccine with a pertussis vaccine added and is thought to decrease the incidence and transmission of pertussis in the respective age group. In Korea, two products are approved by the KOREA FOOD & DRUG ADMINISTRATION, which are ADACEL™ (Sanofi-Pasteur, Totonto, Ontario, Canada) and BOOSTRIX® (GlaxoSmithKline Biologicals, Rixensart, Belgium) for those aged between 11-64. This report summarizes the recommendations approved by the Committee on Infectious Diseases, the Korean Pediatric Society.
Introduction
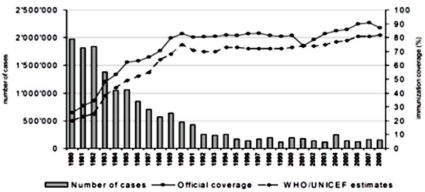
Pertussis is an acute respiratory infection characterized by paroxysmal cough and inspiratory whoop for over 2 weeks. The incidence of pertussis has decreased markedly after the introduction of DTwP/DTaP vaccine ('DTwP/DTaP' hereinafter), but pertussis is still one of important infectious diseases as around 300,000 people are estimated to die of the disease each year throughout the world1) (Fig. 1).
The outbreak of pertussis has decreased significantly after immunization, but according to reports in the U.S., the incidence of pertussis has increased steadily among young infant and among adolescents and adults since the 1980s2-4). This phenomenon is possibly due to the waning immunity since childhood immunization and lack of natural booster events5-8). The immunity against pertussis is maintained only for around 5-6 years after immunization9). The increase of pertussis among adolescents and adults act as a source of pertussis for young infant before immunization with DTaP. Thus, it is important for adolescents and adults to maintain immunity to pertussis in order to decrease the incidence of pertussis in this age group and to protect young infant from pertussis, and Tdap vaccine ('Tdap' hereinafter) was introduced for this purpose.
In the U.S., two products of Tdap were approved in 2005, which are ADACEL™ (Sanofi-Pasteur, Totonto, Ontario, Canada) for those aged between 11-64 and BOOSTRIX®(GlaxoSmithKline Biologicals, Rixensart, Belgium) for those aged between 10-64. In Korea, ADACEL™ was approved in June 2009 and BOOSTRIX® in June 2010 by the KOREA FOOD & DRUG ADMINISTRATION for the prevention of diphtheria, tetanus and pertussis in adolescents and adults aged between 11-64.
The purpose of this statement is to provide the rationale and recommendation for adolescent use of Tdap vaccine in Korea (Box 1).
Pathogen
Pertussis is caused by Bordetella pertussis, a fastidious Gram-negative coccobacillus. It is one of the most highly communicable diseases, attack rates in susceptible household contact range from 70 to 100 percent. The main infection reservoir is adolescents or adults who show a light symptom or do not show any symptom, and the symptom is most severe when the disease occurs in infants younger than 6 months who have not completed the immunization. Pertussis-like syndrome may be caused by adenovirus type 1, 2, 3 and 5, Bordetella parapertussis, Chlamydia trachomatis, etc. Disease caused by Bordetella spp. other than B. pertussis is not prevented by pertussis immunization10).
Epidemiology
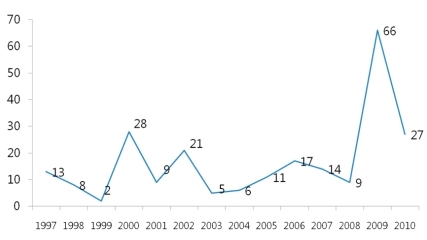
Human is the only host of pertussis. Infection occurs by a direct contact or by respiratory droplets from a coughing patient. The outbreaks of pertussis have decreased markedly with the increase of immunization rate throughout the world (Fig. 1), but are reported intermittently. Despite the high immunization rate since the 1980s, the incidence of pertussis increased steadily in young infants as well as in adolescents and adults3, 4, 11). In particular, adolescents act as a reservoir and spread the disease to young infants with a high risk of complications12, 13). The recent increasing number of reports on the occurrence of pertussis is partially the result of active diagnostic examination and the advance of diagnostic technology. However, the increase of incidence in specific age groups suggests that pertussis may be prevalent continuously in the future if booster vaccination is not performed. Although data are not sufficient on the incidence of pertussis in Korea, according to recent statistics from the Korea Center for Disease Control and Prevention (KCDC), the report of pertussis shows an increasing pattern, so adequate actions should be taken. In Korea, large-scale outbreaks of pertussis have disappeared with the DTwP/DTaP immunization, but small-scale ones have been being repeated since 1995 and around 11.5 cases are being reported each year on the average. However, the accurate incidence is unknown due to the absence of pertussis diagnosis in primary hospitals and sentinel surveillance system, but according to disease surveillance reports by the KCDC, the number of cases increased in 2009 (Fig. 2). In addition, according to domestic data, pertussis was the cause of chronic cough in 2.9% of adults with chronic cough14).
The reasons for the steady rise in the incidence of pertussis include 1) a genetic change in B.pertussis15, 16), 2) the waning immunity, 3) poor management of adolescent and adult pertussis patients. In domestic studies as well, it was found from strains isolated from Korean pertussis patients that pertussis antigens are changing continuously over time17).
Clinical Manifestations
In general, pertussis has an incubation period of 7-10 days. The illness has three stages (catarrhal stage, paroxysmal stage, convalescent stage), and infectivity is highest in the catarrhal stage and falls rapidly afterwards, but transmission is possible for around 3 weeks. A characteristic paroxysmal cough occurs mainly at night and 15 fits around 24 hours on the average, maintaining similar intensity for 2-3 weeks and even up to 10 weeks. The frequency of complications and hospitalization is higher in infants and toddlers younger than 12 months than in older ones13, 18, 19). Among complications, the most common and fatal one is pneumonia caused by secondary bacterial infection. Besides, hypoxia-induced convulsion, encephalopathy, etc. may happen in infants, and paroxysmal cough may cause pneumothorax, nasal bleeding, subdural hematoma, hernia, rectal prolapse, etc.
Pertussis in adolescence shows considerable variation from mild to severe. When classic symptoms do not appear, the disease may not be distinguishable from other respiratory infections. Around half of adolescents diagnosed with pertussis cough for over 10 weeks, 72-100% show symptoms such as paroxysmal cough, dyspnea and sleep disturbance, 50-70% are accompanied with vomiting, 1-2% are hospitalized for pneumonia, etc., and 0.2-1% show symptoms such as convulsion and unconsciousness20, 21). It was found that pertussis was the cause of the cough in 13-20% of adolescents and adults with non-specific cough continuing for over a week22).
Diagnosis
Pertussis is a highly communicable disease, and if one has contacted a pertussis patient and shows a characteristic pattern of cough he/she may be suspected to have pertussis. Bacterial identification through culture study is the most accurate diagnosis method but the culture is quite a complicated process. Recently, the test based on polymerase chain reaction (PCR) is being used increasingly and it is known to show a positive rate 2-3 times higher than that of culture study in typical cases23-25). In a serologic test, the increase of pertussis antibody should be proved in serial serum at the acute stage and the convalescent stage.
Immunization
1. Current state and background of development
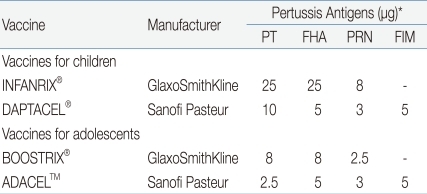
The introduction of Tdap is required in order to decrease the incidence of pertussis in adolescents and adults, to prevent its spread from the older age groups to infants and toddlers, and to maintain herd immunity. It was found that around 76-83% of infant pertussis were from adolescents or adults with waning immunity against pertussis. In both natural infection and immunization, the immunity is not maintained lifelong, so adolescents and adults need to be vaccinated for the maintenance of immunity and now vaccination is available for this age group. In the U.S., two Tdap products for adolescents and adults were approved in 2005 and are being used (Table 1).
These vaccines are similar to Td in terms of the contents of diphtheria and tetanus toxoid, but their content of pertussis vaccine is similar to or reduced to 1/3-1/4 of that of infant DTaP. ADACEL™ (Sanofi-Pasteur) is a 5-component pertussis vaccine, which reduces the pertussis toxin (PT) content to 1/4 from that of DTaP by the same manufacturer but has the contents of filamentous haemagglutinin (FHA), pertactin, and fimbriae 2 and 3 as a same dose, and was approved for those aged between 11-64. BOOSTRIX® (GSK) is a 3-component pertussis vaccine, which reduces 3 pertussis components uniformly to 1/3 among the components of DTaP by the same manufacturer, and was approved for those aged between 10-18 but the applicable age group was expanded recently up to 64 years. In Korea, both of the two products were approved for the prevention of diphtheria, tetanus and pertussis in adolescents and adults aged between 11-64.
2. Immunogenicity
The Tdap products above were approved based on the results of clinical tests showing that they are not inferior to DTaP products of the respective manufacturers in terms of immunogenicity and safety. In clinical trials, the products were found to have immunogenicity and safety against diphtheria, tetanus and pertussis through single vaccination in adolescents. Tdap is administered once because single vaccination with Tdap is considered to induce an antibody response and clinical effect similar to those by three immunizations with DTaP in infants.
3. Vaccination schedule
In case adolescents at the age of 11-18 completed 5 DTaP immunization series during their infancy and childhood, they are vaccinated with Tdap instead of Td once as a booster vaccination against tetanus, diphtheria and pertussis, and the recommended age is 11-12 years. If adolescents at the age of 11-18 were vaccinated with Td but not with Tdap, it is recommended to be vaccinated with Tdap once for maintaining immunity against pertussis and to keep at least 2 years' interval between the vaccination with Td and that with Tdap. However, vaccination with Tdap may be made in a shorter interval in case the risk of pertussis is high (e.g. close contact with a pertussis patient, the prevalence of pertussis, close contact with young infants).
4. Administration and dosage
The vaccine is administered via intramuscularly at a dose of 0.5 mL, and the deltoid muscle is preferred. The vaccine is kept at 2-8℃.
5. Simultaneous administration and interchangeability
Tdap administered during adolescence does not need to be from the same manufacturer as that of DTaP administered during infancy. If all vaccines corresponding to the indications are available in a visit, they can be injected simultaneously at different sites.
6. Adverse events
1) Local adverse events
(1) ADACEL™
Local adverse events observed commonly are pain, edema and erythema, which were observed in 77.8%, 20.9%, and 20.8%, respectively. Severe pain, edema and erythema were observed in 1.5%, 6.4% and 6.0%, respectively. These adverse events are expected to be minimized using an appropriate administration method.
(2) BOOSTRIX®
Local adverse events observed commonly are pain, edema and erythema, which were observed in 75.3%, 21.1% and 22.5%, respectively. Severe pain, edema and erythema were observed in 4.6%, 2.5% and 1.7%, respectively.
2) Systemic adverse events
(1) ADACEL™
Systemic adverse events such as fever, headache and fatigue were observed in 5.0%, 43.7% and 30.2%, respectively, and adverse events such as nausea, vomiting, diarrhea and arthralgia were also observed. Fever of over 39.0℃, severe headache and fatigue were observed in 0.2%, 2.0% and 1.2%, respectively.
(2) BOOSTRIX®
Systemic adverse events such as fever, headache and fatigue were observed in 13.5%, 43.1% and 37.0%, respectively, and gastrointestinal symptoms were observed in 26.0%. Fever of over 39.0℃, severe headache and fatigue were observed in 1.4%, 3.7% and 3.7%, respectively.
3) Severe adverse events
(1) ADACEL™
Severe adverse events within 6 months from the vaccination were observed in 0.9%, but they were reported not to be associated with the vaccine.
(2) BOOSTRIX®
Severe abnormal responses within 6 months from the vaccination were observed in 0.4%, but they were reported not to be associated with the vaccine.
7. Contraindications and Precautions
A serious allergic reaction (e.g. anaphylactic reaction) to the components of vaccine is a contraindication. If encephalopathy of unknown cause happened within 7 days from vaccination with vaccine containing pertussis components, it is a contraindication and in such a case Td should be administered.
In case Guillain-Barre syndrome occurred within 6 weeks after receipt of a tetanus toxoid-containing vaccine in the past, the vaccination should be decided in consideration of risk and benefit. When the subject is under progressive neurologic disorder, the vaccination containing pertussis antigen should be delayed until the disorder is stabilized.
8. Special situations
1) Tetanus prophylaxis indicated for wound management
Should receive Tdap (if not previously given) instead of Td.
2) Lack of availability of Tdap
Should receive Td when Tdap is indicated but not available if the last DTP/DTaP/Td was administered at least 10 y previously. If vaccine was administered less than 10 y previously, immunization can be deferred temporarily if follow-up is likely.
3) The subject has a history of pertussis
Should receive Tdap
4) History of receipt of Td but incomplete pertussis immunization
Should receive catch-up dose of Tdap; a 2 year interval generally is used
5) History of no DTP/DTaP/DT or Td immunization
Should receive catch-up doses of 3 Td-containing vaccines, one of which is Tdap
6) History of receipt of DTP/DTaP/DT or Td but incomplete record
Consider serologic test. If tetanus or diphtheria antibody concentrations are 0.1 IU/ml or greater, presume previous immunization and administer a single dose of Tdap (to be considered the adolescent booster dose)
7) Pregnancy
Pregnancy is not a contraindication to Tdap immunization. But, it should only be given if clinically indicated.
8) Postpartum
Mothers of newborn infants should be given a dose of Tdap as soon as is feasible if they previously have not received Tdap.
9) Children 7 through 10 year of age with history of incomplete childhood DTP/DTaP immunization
Those not fully vaccinated against pertussis and for whom no contraindication to pertussis vaccine exists should receive a single dose of Tdap. Those never vaccinated against tetanus, diphtheria, or pertussis or who have unknown vaccination status should receive a series of three vaccination containing tetanus and diphtheria toxoids. The first of these three doses should be Tdap.
Future prospect
Along with the use of Tdap, related research should be made continuously. It is necessary to monitor the effect and safety of vaccination, and additional studies should be conducted on change in the epidemiology of pertussis in response to vaccination in Korea.