Hu.4-1BB-Fc fusion protein inhibits allergic inflammation and airway hyperresponsiveness in a murine model of asthma
Article information
Abstract
Purpose
4-1BB (CD 137) is a costimulatory molecule expressed on activated T-cells. Repression by 4-1BB is thought to attenuate Th2-mediated allergic reactions. The aim of this study was to investigate the effect of 4-1BB on allergic airway inflammation in a murine asthma model.
Methods
BALB/c mice were sensitized to and challenged with ovalbumin (OVA). Hu.4-1BB-Fc was administered 1 day before the first OVA sensitization or 1 day after the second OVA sensitization. Following antigen challenge, airway responsiveness to methacholine was assessed and bronchoalveolar lavage (BAL) fluid was analyzed. Total immunoglobulin (Ig) E, OVA-specific IgE, IgG1, and IgG2a levels in sera were measured by enzyme-linked immunosorbent assay. Lung pathology was also evaluated.
Results
In mice treated with Hu.4-1BB-Fc before the first OVA sensitization, there was a marked decrease in airway hyperresponsiveness, total cell count, and eosinophil count in the BAL fluid. In addition, Hu.4-1BB-Fc treatment decreased serum OVA-specific IgG1 levels and increased serum IgG2a level significantly compared with the corresponding levels in mice sensitized to and challenged with OVA. Hu.4-1BB-Fc-treated mice also showed suppressed peribronchial and perivascular inflammatory cell infiltration. In contrast, treatment with Hu.4-1BB-Fc 1 day after sensitization had no effect on airway hyperresponsiveness and showed less suppression of inflammation in lung tissue.
Conclusion
Administration of Hu.4-1BB-Fc can attenuate airway inflammation and hyperreactivity in a mouse model of allergic airway inflammation. In addition, administration before sensitization may be more effective. These findings suggest that 4-1BB may be a useful therapeutic molecule against asthma.
Introduction
4-1BB (CD 137), a member of the tumor-necrosis factor receptor (TNFR) superfamily, was originally identified as an inducible costimulatory molecule on activated T-cells1). It is expressed on activated T cells, natural killer (NK) cells, and dendritic cells (DCs), while the ligand of 4-1BB (4-1BBL, TNFSF9) has been detected on activated antigen-presenting cells (APCs) such as B-cells, macrophages and DCs2). 4-1BBL expressed on activated APC binds to 4-1BB that is induced on T-cells, generating positive signals inside T-cells to augment various aspects of immunity3-5). 4-1BB has been originally described as an early costimulator of CD8+ T cells enhancing interleukin (IL)-2 and interferon (IFN)-γ secretion, but more recent data point also to an involvement in CD4+ Th2-mediated responses.
Previous studies have suggested that 4-1BB can protect against tumor or pathogens by inducing activation and expansion of NK cells or CD8+ T cells6,7), block autoimmune disease progression and suppresses rheumatoid arthritis by inducing regulatory T cells8,9). Therefore, 4-1BB has been considered as therapeutic target for various human diseases such as tumor and autoimmune diseases10).
Asthma, one of the most common chronic respiratory diseases, is mediated by inappropriate Th2 cell-mediated immune response. As a costimulatory molecule of TNFR superfamily, 4-1BB upregulates Th2 cell proliferation and promotes airway hyperreactivity, eosinophil infiltration and immunoglobulin (Ig) E production11). Based on these characteristics of 4-1BB, several studies have been conducted to investigate the effect of it against asthma and reported positive results, the suppression of Th2-mediated allergic airway inflammation12-14). However, its effect and mechanism for asthma is not fully understood yet, because of expression of it on diverse cell types as well as its diverse roles and complexities in immune system.
Hu.4-1BB-Fc is a fusion protein of 4-1BB and Fc portion of human IgG1. Its function is known to block the costimulatory activity of 4-1BB. This study was conducted to investigate its effects on the allergic airway inflammation using a mouse model of asthma. Finally it was to find out whether 4-1BB is useful for target molecule against asthma and its mechanism.
Materials and methods
1. Establishment of a mouse model of asthma
A murine model of asthma was established according to a modification of the methods proposed by Jang et al.15). Briefly, female BALB/c mice aged 6 weeks were sensitized by intraperitoneal administration of a mixture of ovalbumin (OVA; 10 µg; grade V, Sigma Inc., St. Louis, MO, USA) and alum (2.25 mg; Imject, Pierce, Rockford, IL, USA). One week after the first sensitization, the mixture was administered intraperitoneally a second time. 5 days later, the mice inhaled 1% OVA using an ultrasonic nebulizer (Nescosonic UN-511, Alfresa, Osaka, Japan) for 3 consecutive days (Fig. 1). The mice were divided into four groups of five mice each: those that received Hu.4-1BB-Fc 1-day before the primary sensitization (group 1), those that received Hu.4-1BB-Fc 1-day after second sensitization (group 2), those that received only saline (instead of OVA) at both sensitization and airway challenge (group 3, negative controls), and those in which asthma was induced (group 4, positive controls). Hu.4-1BB-Fc, Recommbinant Human 4-1BB Fc Chimera (R&D systems, Inc., Minneapolis, MN, USA) is a fusion protein of 4-1BB and Fc portion of human IgG1; A DNA sequence encoding amino acid residues 1 to 186 of the extracellular domain of human 4-1BB was fused to the 6X histidine tagged Fc of human IgG1, via a polypeptide linker. The chimeric protein was expressed in a mouse myeloma cell line, NSO cell (Fig. 1). 20 µg of Hu.4-1BB-Fc was administered intraperioneally per mouse.

Experimental protocol for the induction of allergic asthma and treatment scheme. Four groups of female BALB/c mice were analyzed. Hu.4-1BB-Fc-treated groups (group 1 and group 2) were sensitized to ovalbumin (OVA) with adjuvant via intraperitoneal injections, while the control groups (group 3 and group 4) were sensitized to equivalent amounts of saline and OVA, respectively. Group 1, Hu.4-1BB-Fc-treatment 1-day before the first OVA sensitization; group 2, Hu.4-1BB-Fc-treatment starting 1-day after the second sensitization; group 3, salinesensitized and saline-challenged group; group 4, OVA-sensitized and OVA-challenged group. IP, Intraperitoneal; IgG, immunoglobulin G.
2. Measurement of airway hyperresponsiveness (AHR)
The mice inhaled normal saline and methacholine aerosol (5, 10, 25, and 50 mg/mL) produced by an ultrasonic nebulizer, and a pulmonary function test was performed using an animal airway resistance recorder (body volume change recorder, All Medicus, Seoul, Korea). Twenty-four hours after inhalation of 1% OVA. Solution of each methacholine concentration was diluted using acetyl-β-methacholine chloride (Sigma Aldrich Co., St. Louis, MO, USA). Enhanced pause (Penh) was measured at 10-second intervals for 3 minutes after 3-minute inhalation of saline and each concentration of methacholine.
3. Bronchoalveolar lavage (BAL) and cell fractionation
After measurement of AHR, the trachea was immediately exposed after anesthesia by intraperitoneal administration of ketaminexylazine. BAL was performed through a catheter inserted into the exposed trachea following instillation of normal saline (2 mL) at 37℃. The BAL fluid was centrifuged at 1,500 rpm for 2 minutes at 4℃. After discarding the supernatant, we washed the resultant pellet with phosphate buffered saline (PBS) and resuspended it in 100 µL PBS. Total BAL cell counts were performed using a hemacytometer. BAL cells were stained with Diff-Quick (Sysmex, Kobe, Japan) and a differential cell count was performed on the basis of morphologic criteria and using a light microscope to evaluate at least 200 cells per slide.
4. Measurement of serum immunoglobulin
After blood was withdrawn from the orbital venous plexus, serum was separated from the blood clot by centrifugation at 2,500 rpm for 20 minutes at 4℃. Absorbance of OVA-specific IgE, IgG1 and IgG2a, as well as total IgE, was measured using enzyme-linked immunosorbent assay. Antibodies for OVA-specific IgE and total IgE (Acris antibodies GmbH, Herford, Germany) and IgG1 and IgG2a (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used.
5. Histological examination of murine lung tissue
After BAL, the murine lung was resected, fixed with 10% formalin, and embedded in paraffin. Specimens were cut into 4 µm sections. The microsections were stained with hematoxylineosin and examined under a magnification of ×100. The degree of lung inflammation was evaluated using the previously described methods16). Briefly, lung lesions, including alveolar septal infiltrates, perivascular infiltrates and peribronchial infiltrates, were subjectively graded as 1, 2, 3, or 4 based on a severity scale of minimal, mild, moderate or marked, respectively.
6. Statistical analysis
Groups were compared using the Mann-Whitney U test. All statistical analyses were performed using SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
1. AHR
The mean Penh value at 50 mg/mL methacholine was 3.38±0.80 in group 1 (Hu.4-1BB-Fc treatment before sensitization), 5.32±1.66 in group 2 (Hu.4-1BB-Fc treatment after sensitization), 2.87±0.88 in group 3 (negative control), and 6.53±1.35 in group 4 (positive control). The mean Penh value was significantly decreased in group 1 compared with that in group 4 (P=0.009; Fig. 2), while there was no significant difference between group 2 and group 4 (P=0.251; Fig. 2).
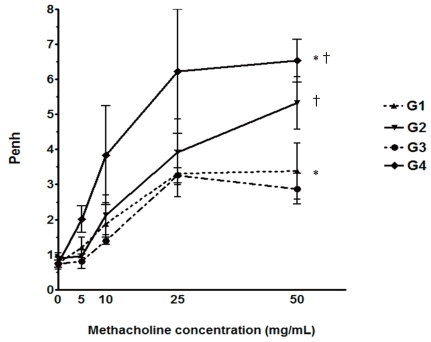
The effects of Hu.4-1BB-Fc on airway hyperresponsiveness. The group administrated Hu.4-1BB-Fc 1-day before the first ovalbumin (OVA) sensitization (group 1) showed significantly less OVA-induced airway hyperresponsiveness to the methacholine challenge as compared to the positive control group (group 4). (*P=0.009 and †P=0.251, as compared with the positive control group). G1, group 1; G2, group 2; G3, group 3; G4, group 4.
2. BAL and cell fractionation
Regarding the mean total number of cells in the BAL fluid, as with AHR, the total number was less in group 1 (0.79±0.37×105/mL) compared with group 4 (1.14±0.42×105/mL) even though it was not significant (P=0.095; Fig. 3). The mean percentage of eosinophils in the BAL fluid was 0.80±0.40% in group 1, 1.38±0.33% in group 2, 0.01±0.00% in group 3, and 8.86±3.07% in group 4 (Fig. 4). Compared with group 4, there were significant differences in the percentage of eosinophils in groups 1 and 2 (P=0.009, P=0.008) respectively (Fig. 4).
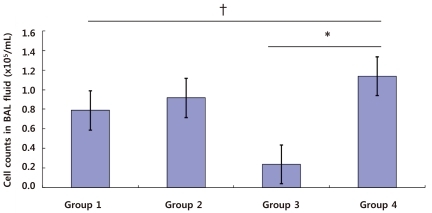
Total cell counts in bronchoalveolar lavage (BAL) fluid. There was a significant difference between the negative control group (group 3) and the positive control group (group 4) (*P<0.01). The group administered Hu.4-1BB-Fc 1-day before first ovalbumin sensitization (group 1) had lower total cell counts in the BAL fluid than the positive control group (group 4) (†P=0.095).
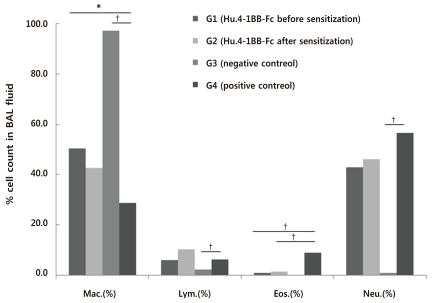
Differential cell count in bronchoalveolar lavage (BAL) fluid. Twenty-four hours after the last ovalbumin challenge mice were killed and tracheas were cannulated for BAL. Cells from the BAL fluid were counted, spun onto glass slides, and stained with Diff-Quick before microscopy (*P<0.05 and †P<0.01). G1, group 1; G2, group 2; G3, group 3; G4, group 4; Mac., Macrophage; Lym., Lymphocyte; Eos., Eosinophil; Neu., Neutrophil.
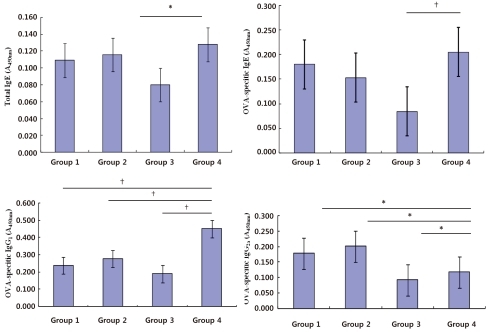
3. Serum levels of total IgE and OVA-specific IgE
Mean total IgE levels (A450nm) were 0.08±0.00 in group 3 and 0.12±0.00 in group 4 (P<0.05; Fig. 5). Compared with group 4, the total IgE level was less in group 1 (0.11±0.03) and 2 (0.12±0.0.03) but the difference was not statistically significant. And the differences of mean OVA-specific IgE levels were 0.18±0.01 in group 1, 0.15±0.03 in group 2, 0.08±0.01 in group 3, and 0.20±0.06 in group 4. The levels were not statistically significant between groups 1 and 4 neither between group 2 and group 4.
4. Serum levels of OVA-specific IgG1 and IgG2a
Mean serum levels of OVA-specific IgG1 (A450nm) were 0.19±0.02 in group 3 and 0.45±0.01 in group 4; the differences were statistically significant (P<0.01; Fig. 5). The OVA-specific IgG1 levels were 0.23±0.00 in group 1 (P=0.09) and 0.27±0.01 in group 2 (P=0.09) and they were significantly lower compared with group 4. Mean serum levels of OVA-specific IgG2a (A450nm) were 0.17±0.02 in group 1 and 0.20±0.04 in group 2. Compared with group 4 (0.11±0.01), the levels in group 1 and 2 were significantly higher (P=0.014, P=0.020, respectively; Fig. 5).
5. Histologic examination of murine lung tissue
Upon hematoxylin-eosin staining, peribronchial and perivascular infiltration of eosinophils was significantly heavier in group 4 compared with group 3 (Fig. 6A, B). Compared with group 4, there was significantly less heavier in eosinophil infiltration in group 1 (Fig. 6C) while group 2 was not significantly different (Fig. 6D).
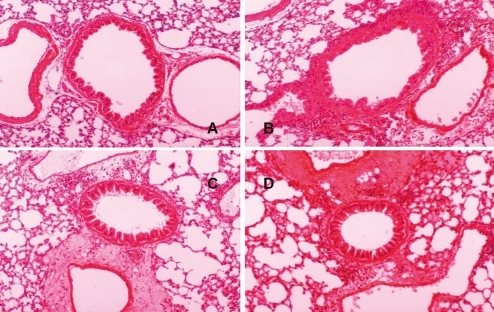
Effects of Hu.4-1BB-Fc on pulmonary inflammation. Representative lung sections from 2 groups of Hu.4-1BB-Fc-treated mice ([C] group 3, before sensitization; [D] group 4, after sensitization). (A) group 3, negative control and (B) group 4, positive control (A-D, H&E stain, original magnification, ×100).
Discussion
Asthma, which has increased substantially in prevalence recently, is characterized by airway hypereactivity, chronic airway inflammation with lung eosinophilia, and increased serum IgE level17). It is considered to be a result of an inappropriate Th2 cell-mediated immune reaction. Up to now, current treatment, steroid is known to be only symptomatic but not to modify the disease18).
The present study showed Hu.4-1BB-Fc attenuated allergic inflammation and AHR using a murine model of asthma. Hu.4-1BB-Fc treatment decreased AHR and eosinophilic airway inflammation in OVA-sensitized BALB/c mice. These results are consistent with previous studies using therapeutic strategy of 4-1BB and suggested that 4-1BB would be a target molecule for controlling asthma12-14,19).
In this experiment, AHR was significantly decreased at 50 mg/mL methacholine (maximum concentration) in group treated with Hu.4-1BB-Fc before sensitization compared with positive control group. Percentage of eosinophils was significantly lower in group treated with Hu.4-1BB-Fc before and after sensitization compared with positive control group. Hematoxylin-eosin staining revealed that peribronchial and perivascular infiltration of inflammatory cells was significantly decreased by treatment with Hu.4-1BB-Fc before sensitization but not treatment with Hu.4-1BB-Fc after sensitization. Therefore, Hu.4-1BB-Fc treatment may have a better effect when it is administered before sensitization rather than after sensitization, which suggests that Hu.4-1BB-Fc treatment may be effective in the prevention of asthma.
Total IgE and OVA-specific IgE levels, however, were not significantly decreased in Hu.4-1BB-Fc treated mice though they were slightly decreased. In contrast, OVA-specific IgG1 level was significantly decreased while OVA-specific IgG2a was significantly increased in Hu.4-1BB-Fc treated mice. These findings suggest that Hu.4-1BB-Fc treatment could affect Th1/Th2 balance. Previous studies demonstrated that 4-1BB reduced Th2 cytokine production (IL-4, IL-5) and increased Th1 cytokine IFN-γ13,14). Up to date, 4-1BB's role in Th2-mediated immune responses has not been well defined, however, these finding implies that manipulation of 4-1BB may suppresses Th2 response while it augments Th1 response.
4-1BB is a one of T-cell costimulatory molecules, which are now considered to be optimal targets for the treatment of allergic airway disease10). One step of leading to chronic inflammatory airway reaction is that naive T cells recognizing the allergen for the first time become activated and undergo a differentiation into Th2 effector T cells. The differentiation of naive T cells or reactivation of CD4+ T cells into Th2 effector T cells depends on 2-way communication between the APCs and T cells. While a first signal gives antigen specificity by T-cell recognition of major histocompatibiltiy class II at surface of APCs, a second nonspecific costimulatory signal is required by T cell to become fully activated11,20). In general, costimulatory molecules are divided into 2 main families: molecules from the B7 family, such as CD28 or cytotoxic T lymphocyte associated antigen 4 (CTLA-4), and from the TNFR superfamily such as 4-1BB. They have been known for tuning of the T-cell response by mediating both stimulatory as well as inhibitory signals. We previously reported that blockade of CD28 pathway using cytotoxic T lymphocyte associated antigen 4-immunoglobulin (CTLA-4-Ig) inhibited eosinophilic airway inflammation and AHR in OVA-sensitized and airway challenged mice15). Although the relative contributions of costimulatory molecule family, TNFR including 4-1BB for the development and function of Th2 effector cells in not fully understood yet, it is clear that all these molecules collectively represent the master switches for the interaction of T cells with DCs, B cells, and other cells of the Th2 immune reaction. Therefore, it can be expected that a blockade of the costimulatory molecules would attenuate the Th2-mediated allergic reaction20).
Previous studies regarding a therapeutic strategy of 4-1BB reported the positive results12-14,18). Engagement of 4-1BB inhibited the development of experimental allergic conjunctivitis in mice12). Sun et al.13) showed inhibition of Th2-Mediated allergic airway inflammatory disease by 4-1BB costimulation. 4-1BB (CD137)-mediated immunotherapy for allergic asthma prevented asthma in OVA-sensitized BALB/c mice14). However, all these studies used agonistic anti-4-1BB monoclonal antibody12-14,18). Stimulation of the 4-1BB pathway suppressed allergen-specific IgE production and AHR, through deletion of allergen-specific Th2 cells18). Therefore, it seems that 4-1BB could stimulate some regulatory signal to suppress the Th2 response as well21). In a different way, the present study also demonstrated the blockade of 4-1BB may attenuate an allergic inflammation. The exact reason why the different methods showed similar results is unclear at present.
Another consideration about 4-1BB as therapeutic target is that whether the manipulation of a single pathway would achieve the desired therapeutic effects. It is therefore conceivable that an efficient suppression of an exaggerated Th2 immune response would only be achieved with a simultaneous manipulation of several costimulatory pathways involved in this reaction. Further study for such combinatorial blockade would achieve a clearly Th2-biased and therefore more specific immune suppression compared with that seen broadly acting agents, such as corticosteroid or cyclosporine A.
In conclusion, Hu.4-1BB-Fc treatment, especially before sensitization, can attenuate airway hyperreactivity and eosinophilic airway inflammation in a murine model of asthma. These findings suggest that 4-1BB could be a good target molecule for treating asthma. Although it is postulated that its effects are through the modification of Th1/Th2 balance, the exact mechanism is still need to be elucidated.
Acknowledgement
This study was supported by Sam-A Academic Award (2003) of Korean Pediatrics Society.