Clinical characteristics of 2009 pandemic influenza A (H1N1) infection in children and the performance of rapid antigen test
Article information
Abstract
Purpose
In autumn 2009, the swine-origin influenza A (H1N1) virus spread throughout South Korea. The aims of this study were to determine the clinical characteristics of children infected by the 2009 H1N1 influenza A virus, and to compare the rapid antigen and real-time polymerase chain reaction (PCR) tests.
Methods
We conducted a retrospective review of patients ≥18 years of age who presented to Soonchunhyang University Hospital in Seoul with respiratory symptoms, including fever, between September 2009 and January 2010. A real-time PCR test was used to definitively diagnose 2009 H1N1 influenza A infection. Medical records of confirmed cases were reviewed for sex, age, and the time of infection. The decision to perform rapid antigen testing was not influenced by clinical conditions, but by individual factors such as economic conditions. Its sensitivity and specificity were evaluated compared to real-time PCR test results.
Results
In total, 934 patients tested positive for H1N1 by real-time PCR. The highest number of patients (48.9%) was diagnosed in November. Most patients (48.2%) were aged between 6 and 10 years. Compared with the H1N1 real-time PCR test results, the rapid antigen test showed 22% sensitivity and 83% specificity. Seventy-eight patients were hospitalized for H1N1 influenza A virus infection, and fever was the most common symptom (97.4%).
Conclusion
For diagnosis of 2009 H1N1 influenza A virus infection, the rapid antigen test was inferior to the real-time PCR test in both sensitivity and specificity. This outcome suggests that the rapid antigen test is inappropriate for screening.
Introduction
The influenza A virus was prevalent from late fall until early spring every year since it caused the Spanish flu in 1918, which at the time resulted in excessive mortality1). With the advent of the 21st century, a novel influenza A variant of swine origin - H1N1 - appeared which spread to humans and then manifest rapidly across the world since it began in America and Mexico in March 20091-3). Eventually, on June 11, 2009, the World Health Organization raised the worldwide pandemic alert level to phase 6, and announced it was meaningless to continue the count. As of November 2009, 622,482 patients had been definitively diagnosed with the 2009 H1N1 influenza A virus4). In South Korea, the first 2009 H1N1 influenza A patient was reported in May 2009 and since then it became prevalent across the nation and caused 243 deaths by 27 February, 20105).
Children and young people are more susceptible to the 2009 H1N1 influenza A virus and patients may present with general symptoms similar to those of seasonal influenza. However, digestive symptoms tend to be more common, which are known to be mild2,5).
Influenza can be prevented by administering antiviral agents and practising isolation, which premises rapid diagnosis. However, during the 2009 H1N1 influenza A pandemic, many institutions failed to identify proper diagnostics and thus used the rapid antigen test as a temporary measure, until the government recommend real-time polymerase chain reaction (PCR)6). The sensitivity of the rapid antigen test for the 2009 H1N1 Influenza A virus has been reported to lie between 10 and 70%, whereupon it remains controversial as to whether it is appropriate for screening7-9).
The aims of this study were to analyze the clinical characteristics of children hospitalized with H1N1 infection, and to compare between the results of the real-time PCR and rapid antigen tests.
Materials and methods
This retrospective study was conducted on patients aged 18 years and below diagnosed with suspected the 2009 H1N1 influenza A virus infection, who experienced fever of ≥37.8℃, upper and lower respiratory symptoms such as cough, sore throat, nasal discharge, nasal congestion, and who presented to the Soonchunhyang University Seoul Hospital between September 2009 and January 2010. All 934 patients were definitively diagnosed with the 2009 H1N1 influenza A by using the AdvanSure Real-Time PCR Kit (LG Life Sciences Ltd, Seoul, Korea). Statistical analysis was performed on patients who received definitive diagnoses in relation to sex, age and period, by using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). In addition, a retrospective analysis was performed on the medical records of all 78 patients admitted to the pediatric ward, whereof data were collected in relation to sex, age, length of admission, chief complaints and the frequency of complications.
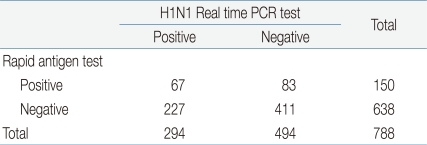
Of all patients definitely diagnosed with the 2009 H1N1 influenza A virus infection, 788 underwent the rapid antigen test by using a test kit (SD Influenza Antigen Kit, Standard Diagnostics Inc., Yongin, Korea). The results of rapid antigen test were used to determine diagnostic sensitivity, specificity, false positive rate, positive predictability and negative predictability. Specimens were taken from the nasal cavities of patients by using sterile swabs irrespective of age. The specimens collected were tested by the reagent offered by the manufacturer. In case of the H1N1 real-time PCR test, AdvanSure Influenza A Kit was used irrespective of age. Nasopharyngeal aspirates were transferred into sterilized tubes, and were tested in accordance with the manufacturer's protocol.
Results
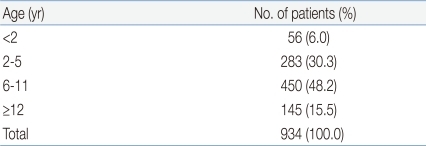
In total, 934 patients were tested positive for the 2009 H1N1 influenza A virus by real-time PCR. They were therefore definitively diagnosed with the 2009 H1N1 influenza A virus infection. The patient population was comprised of 492 (52.8%) males and 441 (47.2%) females. Patients aged between 6 and 11 accounted for the largest patient population, at 450 (48.2%), followed by patients aged between 2 and 5 (283, 30.3%), between 12 and 18 (145, 15.5%) and patients less than 2 (56, 6%). The median age was 7 (Table 1). In relation to the time of diagnosis, most patients (457, 48.9%) were diagnosed in November 2009, followed by December 2009 (233, 23.9%), October 2009 (207, 22.2%), January 2010 (46, 4.9%) and September 2009 (1, 0.1%) (Fig. 1).
Patients Diagnosed with the 2009 H1N1 Influenza A Virus from September 2009 to January 2010 According to Age

Frequency of presenting symptoms in hospitalized patients with confirmed 2009 H1N1 influenza A infection.
During the same period, a total of 78 patients aged 18 and below were hospitalized with the 2009 H1N1 influenza A virus. The average age was 6.8 and the average length of hospital stay was 4.8 days. The number of male and female patients was respectively 35 and 43. In relation to symptoms, fever was the most common (97.4%), followed by cough (83.3%), nasal discharge (29.4%), headache (19.2%), abdominal pain (14.1%), myalgia (12.8%), sore throat (10.0%), dyspnea (9.0%) and diarrhea (3.8%) (Fig. 2). Regarding complications, bronchopneumonia, acute gastroenteritis and lobar pneumonia occurred in respectively 52 (66.6%), 10 (12.8%) and 6 (7.7%) patients. In addition, acute lymphadenitis (1, 0.1%), meningitis (1, 0.1%) and acute laryngotracheobronchitis (1, 0.1%) occurred at same frequencies.

Frequency of symptom presentation in patients with confirmed 2009 H1N1 influenza A hospitalized from September 2009 to January 2010 (n=78). Abd., abdominal.
Out of 934 patients diagnosed with the 2009 H1N1 influenza A virus infection, 788 underwent the rapid antigen test. One hundred and fifty patients showed positive results and 638 patients showed negative results. Based on the results of H1N1 real time PCR test, the rapid antigen test demonstrated a sensitivity of 22.7%, a specificity of 83.1%, a false positive rate of 16.8%, a false negative rate of 77.2%, a positive predictability of 44.6% and a negative predictability of 64.6% (Table 2).
Discussion
The 2009 H1N1 influenza A was prevalent throughout South Korea during the autumn of 2009 and caused a large number of morbidity and mortality. However, it was not so virulent and thus the number of serious cases and deaths considering the number of patients were not high. Remarkably, patients aged 18 and below constituted a large proportion of patients, which might have been caused by prolonged exposure at nurseries and schools2,3,5). Patients diagnosed with the 2009 H1N1 influenza A virus infection at our institution were mostly pre-school or school children aged between 6 and 11. In other studies that included patients of all ages, morbidity was highest in patients aged between 5 and 19 years3,10). Likewise in another study conducted at a hospital located in South Korea during the same period, patients aged between 0 and 9 infected with the 2009 H1N1 influenza A experienced the highest morbidity. The median age was 132). The 2009 H1N1 influenza A incidence tended to rise in September and abate after November, but in reality infection could occur before September but there had been no available diagnostics until then. Moreover, the estimated number of patients may be greater than the published number of patients. Likewise, this study excluded patients diagnosed with the 2009 H1N1 influenza A virus infection through the use of respiratory-virus multiplex PCR before September 2010. In addition, there were many patients who experienced symptoms suggestive of the 2009 H1N1 influenza A virus infection and underwent rapid antigen tests, but did not undergo tests for definitive diagnosis due to financial pressures. Thus, the number of patients would be higher than the published number. The rapid antigen test was performed mostly on outpatients and emergency patients. It was seen as a screening test until its usefulness was proven. In this study, its sensitivity was 22.7%. In other studies, its sensitivity for the 2009 H1N1 influenza A virus ranged between 10 and 70%11,12). The rapid antigen test is inadequate as a screening test for the 2009 H1N1 influenza A virus, because of its low sensitivity, which renders differentiation between the 2009 H1N1 influenza A and seasonal influenza challenging. On the other hand, it can be performed more easily and conveniently than viral culture and PCR, does not impose heavy financial pressure on patients compared with H1N1 real-time PCR, and capable of generating results in a short-turn around time. In addition, it gives a low false positive rate with high specificity, which reduces unnecessary use of antibiotics and antiviral agents and indiscriminate performance of H1N1 real-time PCR. Taken together, it may be considered to be the initial test.
The most common presenting complaints of children and young people infected by the 2009 H1N1 influenza A virus were fever and cough, which did not differ from those of adults2). Also, such symptoms were similar to those of respiratory viral diseases. Moreover, Symptoms such as fever, cough and sore throat showed high sensitivity not only in patients who were definitively diagnosed with seasonal influenza (64 to 78%), but also in patients with the 2009 H1N1 influenza A (87.9%)5,13-15). Regarding complications, bronchopneumonia was the most common. The length of hospital stay was not longer in contrast with respiratory viral diseases16). In a previous study, the sensitivity of the rapid antigen test was analyzed against age, and it demonstrated highest sensitivity in patients aged less than 2 years6). In several studies, sensitivity was defined as time taken for patients to manifest their symptoms. It was shown that the sensitivity tended to be significantly low 48 hours after subjects developed symptoms17,18). In this regard, further studies are required to validate the conclusions. Some patients infected with the 2009 H1N1 influenza A virus experienced serious vomiting and stomach ache, distinct to patients with seasonal influenza12,19,20).
The 2009 H1N1 influenza A pandemic has offered clinicians invaluable experience. From now on there is a need to be fully prepared for pandemics through effective application of the rapid antigen test, real-time PCR and rapid diagnoses, systematic treatments and vaccinations.