Effect of respiratory syncytial virus infection on regulated on activation, normal T-cells expressed and secreted production in a murine model of asthma
Article information
Abstract
Purpose
Synthesis of regulated on activation, normal T-cells expressed and secreted (RANTES) in the airway has previously been shown to be elevated after respiratory syncytial virus (RSV) infection. However, since few studies have examined whether RSV-infected asthma patients express a higher level of RANTES than do normal individuals, we used a murine model of asthma to address this question.
Methods
We prepared Dermatophagoides farinae-sensitized mice as an asthma model, and then infected them with RSV and analyzed the changes in airway responsiveness and the cell populations and cytokine levels of bronchoalveolar lavage fluid.
Results
RANTES synthesis increased in response to RSV infection in both control mice and in asthma model (D. farinae) mice. However, there was no significant difference in the amount of RANTES produced following RSV infection between control and D. farinae mice. RSV infection affected neither interferon-γsynthesis nor airway responsiveness in either control or D. farinae mice.
Conclusion
RSV infection did not induce more RANTES in a murine model of asthma than in control mice.
Introduction
Introduction Asthma is a chronic inflammatory disease of the airway characterized by airway hyper-responsiveness and airflow obstruction. As a common disease affecting approximately 300,000,000 people worldwide1), asthma is of great interest, and a significant amount of research into asthma has been carried out. This has led to the development of a number of treatment options for asthma that have improved the control of asthma symptoms compared with what was achievable in the past2). Nevertheless, acute exacerbation of asthma remains an important issue affecting the management of this disease. Viral respiratory tract infection is the most common cause of acute asthma attacks and has been associated with 80% and 50% of these attacks in children and adults, respectively3). However, the mechanism by which virus infection induces acute exacerbation of asthma is unclear. While a number of viruses may infect the respiratory tract, respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infection, particularly in children during the winter. As such, RSV is an important causative agent of the induction of acute asthma attacks4).
Regulated on activation, normal T-cells expressed and secreted (RANTES) is a chemokine that stimulates eosinophil activation. As increased infiltration of eosinophils into the asthmatic airway is closely associated with asthma exacerbation and severity, the involvement of this chemokine in asthma is of interest5). RANTES synthesis has previously been shown to be elevated in the airway after RSV infection6); however, one study has shown that the increase of RANTES expression in response to RSV infection did not differ between a mouse model of asthma and control mice7). However, this was the sole report on this subject that we could find, the result was determined as a minor part of a study designed to examine recurrent RSV infection and repeated allergen exposure, and the amount of RANTES was determined in lung homogenate rather than in bronchoalveolar lavage (BAL) fluid. Therefore, as few studies have thoroughly examined whether asthma patients express higher levels of RANTES in response to RSV infection than do normal individuals, we used a murine model of asthma to address this question.
Materials and methods
1. Preparation of Dermatophagoides farinae-sensitized mouse asthma model
A murine model of asthma was created using 4- to 6-week-old female BALB/c mice8), which were treated according to the regulations of the internal review board of the Clinical Medical Research Center, The Catholic University of Korea School of Medicine. Briefly, animals were sensitized on days 0 and 14 by intraperitoneal injection of 250 µg of crude extract of D. farinae (Arthropods of Medical Importance Resource Bank, Seoul, Korea) dissolved in 200 µL phosphate-buffered saline (PBS) containing 2 mg aluminum potassium sulfate. On days 14, 15, and 16, mice were anesthetized with isoflurane (Vedco, St. Joseph, MO, USA) and 50 µg of D. farinae dissolved in 50 µL PBS was introduced into the nasal cavity by inhalation (Fig. 1). The successful creation of the murine asthma model was assessed by examining the airway responsiveness, type 2 cytokine expression levels and numbers of eosinophils in BAL fluid, and lung histopathology of the mice.
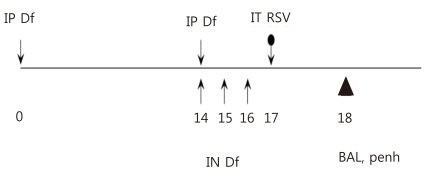
Study design. Dermatophagoides farinae (Dermatophagoides farina, Df)-sensitized mice were immunized with Df plus alum by intraperitoneal injection (IP) once on days 1 and 14. Two weeks later, these mice were inoculated intranasally (IN) once daily with Df for 3 consecutive days (days 14 to 16). Df-sensitized mice were infected with respiratory syncytial virus (RSV) 24 hours after the final Df inoculation. Penh values and cytokine levels in bronchoalveolar lavage (BAL) fluid were measured 24 hours after RSV infection. IT, intratracheally.
2. RSV preparation and infection of mice
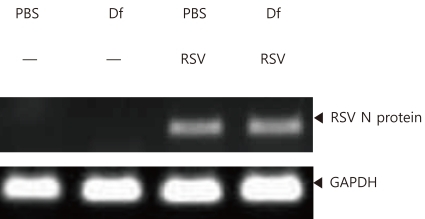
RSV A2 strain (American Type Cell Culture, ATCC, Rockville, MD, USA) was used to infect HeLa cells (KCLB No. 10002, Korean Cell Line Bank, Seoul, Korea) in 96-well plates; the infection titer was determined by measuring the tissue culture infective dose 50% (TCID50). On day 17, 50 µL of RSV in PBS at a concentration of 5×106 TCID50/mL was administered into the trachea of mice anesthetized with a small dose of a mixture of xylazine and tiletamine/zolazepam. Infection was confirmed by reverse transcriptase polymerase chain reaction (RT-PCR) to detect RSV N protein mRNA in the lung tissues of the infected mice as previously decribed8).
3. Reverse transcriptase polymerase chain reaction
Total RNA was extracted from lung homogenate using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The isolated RNA was treated with DNase (Invitrogen) and cDNA was synthesized using SuperScript reverse transcriptase (Invitrogen). PCR amplification using RSV-1005 (Maxim Biotech, South San Francisco, CA, USA) was performed on a 1-µL cDNA sample; the optimum annealing temperature and number of amplifications were determined for each primer pair, and PCR products were obtained within the linear range of amplification. To control for sample-to-sample variation, the cDNA concentration of each sample was adjusted to comparable levels of GAPDH transcripts prior to PCR amplification. The resulting PCR products were separated by electrophoresis on 2% agarose gels, stained with ethidium bromide, and visualized under ultraviolet light. Bands were quantified using densitometry analysis (Gel Doc, Bio-Rad Laboratories Inc., Hercules, CA, USA).
4. Experimental design
On day 17, PBS-inoculated and D. farinae-sensitized asthma model mice were infected with RSV or left uninfected. Six mice per group were used. BAL fluid was collected and airway resistance measured on day 18 (Fig. 1).
5. Determination of airway responsiveness
Airway responsiveness was measured with an OCP3000 instrument (Allmedicus, Anyang, Korea) using 4 different concentrations of methacholine (12.5 mg/mL, 25 mg/mL, 50 mg/mL, and 100 mg/mL). An Ultra-Neb ultrasonography nebulizer (3650p; Pulmo-Aide LT Lompressor, Somerset, PA, USA) was used to administer an aerosolized 3-mL volume of each concentration of methacholine over a period of 3 minutes. The enhanced pause (Penh) variable was also measured over a period of 3 minutes.
6. Preparation of BAL fluid
After measuring airway responsiveness, the main bronchus was ligated with thread and BAL performed on the whole lung using a 22-gauge needle inserted into the trachea. PBS (1 mL) was injected and BAL fluid harvested once and centrifuged at 2,000 rpm for 10 minutes at 4℃. Supernatants were stored at -70℃.
7. Analysis of cells in BAL fluid
Cell precipitates were suspended in 0.4 mL PBS and a 10-µL volume mixed with 10 µL of trypan blue solution (Gibco, Grand Island, NY, USA). The total number of cells present in each sample was counted using a hemocytometer. The concentration of cells was adjusted to 1×106 cells/mL, and slides were prepared using a cytocentrifuge (Cytospin 2; Shandon, Runcorn, UK). The slides were stained with Diff-Quik (Sysmex Corporation, Kobe, Japan), and cells (500 minimum) were counted at ×400 magnification in order to determine the differential counts of macrophages, eosinophils, lymphocytes, and neutrophils.
8. Analysis of RANTES and interferon-γ (IFN-γ) in BAL fluid
The concentrations of RANTES and IFN-γ present in the supernatants of the BAL fluid were measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Duoset, MN, USA). All procedures were performed according to the manufacturer's instructions.
9. Statistical analysis
Values are presented as mean±standard error of mean (SEM). Statistical validation of each variable was performed by the Mann-Whitney U test using SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA). P values lower than 0.05 were considered statistically significant.
Results
1. Verification of the murine model of asthma
1) Increased airway resistance
We first investigated the airway responsiveness, as measured by Penh, of our murine model of asthma (D. farinae) compared with control (PBS) mice and observed that the D. farinae mice had higher Penh values than the PBS mice (Fig. 2A).
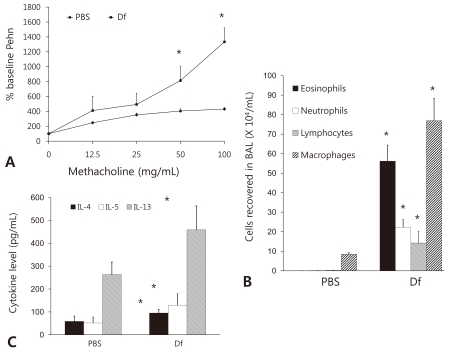
Confirmation of the murine model of asthma. (A) Penh values of the airway response to methacholine in the phosphate-buffered saline (PBS) and asthma (Dermatophagoides farina , Df) groups. (B) Differential cell counts in bronchoalveolar lavage (BAL) fluid collected from the murine model of asthma (Df) and control mice. (C) Cytokine levels in bronchoalveolar lavage (BAL) fluid collected from Df and PBS mice. The levels of interleukin (IL)-4, IL-5, and IL-13 were analyzed by enzyme-linked immunosorbent assay. Data shown represent the mean±SEM (n=6 per group). *P<0.05 vs. PBS.
2) Eosinophilic inflammation in BAL fluid
The number of eosinophils was found to be significantly higher in BAL fluid harvested from D. farinae mice than in that harvested from PBS mice (Fig. 2B). The levels of other leukocytes, such as neutrophils, lymphocytes, and macrophages, were likewise found to be significantly higher in the BAL fluid from D. farinae mice than in that from PBS mice (Fig. 2B).
3) Increased levels of type 2 cytokines in BAL fluid
The expression levels of interleukin (IL)-4, IL-5, and IL-13 in BAL fluid as measured by ELISA were found to be significantly higher in the fluid from the D. farinae mice than in that from the PBS mice (Fig. 2C).
4) Eosinophilic inflammation of lung tissue
Lung inflammation was examined in hematoxylin and eosin (HE)-stained lung tissue harvested either at the time of either the primary RSV inoculation or the final D. farinae injection. Representative photomicrographs showing features of the HE-stained tissues are shown in Fig. 3. Infiltration of the tracheal and bronchiolar epithelia by numerous inflammatory cells, particularly eosinophils, was observed in D. farinae mice. Instability and hyperemia of airway epithelial cells, edema, and the metaplasia of many pulmonary epithelial cells to goblet cells were observed in D. farinae mice (Fig. 3B) but not in PBS mice (Fig. 3A).

Respiratory syncytial virus (RSV)- and Dermatophagoides farinae-induced lung inflammation in a murine model of asthma. Lung tissue was removed at the time of the primary RSV inoculation or the final D. farinae injection and stained with hematoxylin and eosin (H&E). Representative photomicrographs from each group (n=6 per group) are shown as follows: (A) Phosphate-buffered saline (PBS) mice, (B) D. farinae mice, (C) PBS mice infected with RSV, (D) D. farinae mice infected with RSV (A-D, H&E, ×200).
2. Assessment of RSV infection in the murine lung
RSV infection was assessed by performing RT-PCR on homogenized lung tissues from PBS and D. farinae mice. As RSV N protein mRNA was detected in these tissues following RSV infection, infection was determined to have been successful (Fig. 4).
3. RANTES production in RSV-infected mice
RANTES expression was first assessed by performing RT-PCR on homogenized lung tissues. Higher levels of RANTES mRNA were detected in both PBS mice and D. farinae mice infected with RSV than in uninfected mice (Fig. 5A). The amount of RANTES present in BAL fluid was also measured by ELISA. As for the mRNA levels, the levels of RANTES protein expressed by the uninfected PBS and D. farinae mice were comparable and increased to a similar extent following RSV infection (P<0.05, Fig. 5B). This result suggests that RSV infection leads to higher expression of RANTES. However, no significant difference in RANTES expression was observed between the RSV-infected PBS mice and the RSV-infected D. farinae mice (Fig. 5B).
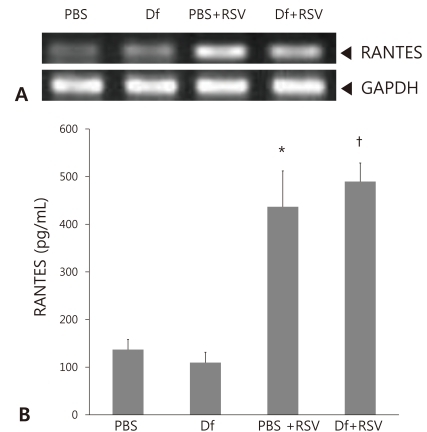
Regulated on activation, normal T-cells expressed and secreted (RANTES) expression in BALB/c mice. (A) RANTES mRNA was amplified by reverse transcriptase polymerase chain reaction (RT-PCR) of lung tissue harvested from uninfected and respiratory syncytial virus (RSV)-infected phosphate-buffered saline (PBS) mice and Dermatophagoides farina (Df) mice. (B) The concentration of RANTES in bronchoalveolar lavage fluid from uninfected and RSV-infected PBS and Df mice was analyzed by enzyme-linked immunosorbent assay. Data shown represent the mean±SEM (n=6 per group). *P<0.05 vs. PBS. †P<0.05 vs. Asthma.
4. IFN-γ production in RSV-infected mice
The amounts of IFN-γ in the BAL fluid from RSV-infected and uninfected PBS and D. farinae mice were measured by ELISA. No differences in IFN-γ levels were observed among the groups (Fig. 6A).

Interferon-γ (IFN-γ) expression, Penh values, and immune cell numbers. (A) IFN-γ levels in bronchoalveolar lavage (BAL) fluid from uninfected and respiratory syncytial virus (RSV)-infected phosphate-buffered saline (PBS) and Dermatophagoides farina (Df) mice. Levels of IFN-γ were analyzed by enzyme-linked immunosorbent assay. (B) Penh values of the airway response to methacholine in uninfected and RSV-infected PBS and Df mice. (C) Differential counts of eosinophils, neutrophils, lymphocytes, and macrophages in BAL from uninfected and RSV-infected PBS and Df mice. Data represent the mean±SEM of 5 independent experiments (n=6 per group). *P<0.05 vs. PBS mice. †P<0.05 vs. Df mice.
5. Alteration of airway responsiveness following RSV infection
No significant differences in airway responsiveness were observed between PBS mice and RSV-infected PBS mice or between D. farinae mice and RSV-infected D. farinae mice (Fig. 6B).
6. Alteration of the cell composition of BAL fluid in response to RSV infection
The number of eosinophils detected in the BAL fluid obtained from RSV-infected PBS mice was significantly increased compared with that of uninfected PBS mice. In contrast, the number of eosinophils was significantly reduced in our murine model of asthma (D. farinae mice) infected with RSV infection compared with that of uninfected D. farinae mice (Fig. 6C). A similar trend was observed when macrophages were examined: the number of macrophages present in the BAL fluid was significantly increased in RSV-infected PBS mice compared with uninfected PBS mice but significantly reduced in RSV-infected D. farinae mice compared with uninfected D. farinae mice (Fig. 6C). When the number of neutrophils present in the collected BAL fluid was examined, significantly higher numbers of these cells were detected in the presence of RSV infection in both PBS and D. farinae mice (Fig. 6C). Finally, although the number of lymphocytes detected in the BAL fluid of PBS mice was increased in the RSV-infected mice, no difference in lymphocyte number was detected between uninfected and RSV-infected D. farinae mice (Fig. 6C).
7. Histological changes in lung tissue
Neutrophils and lymphocytes in particular were observed to have infiltrated the tracheal and bronchiolar epithelium of RSV/PBS mice (Fig. 3C). The pulmonary inflammation found in the D. farinae mice and RSV-PBS mice was also observed in the RSV/D. farinae mice (Fig. 3D).
Discussion
Our results indicate that RANTES synthesis is increased in response to RSV infection in both PBS mice and in our murine model of asthma (D. farinae mice) but that there is no significant difference between these groups of mice in the amount of RANTES produced following RSV infection. In contrast, RSV infection affected neither IFN-γ synthesis nor airway responsiveness in either of these groups. In D. farinae mice, the number of neutrophils in BAL fluid increased and the number of eosinophils decreased with RSV infection.
RSV infection has previously been reported to stimulate the secretion of RANTES in lung tissues in a murine model of asthma7). RANTES has also been found to be released in greater amounts from RSV-infected airway epithelial cells than from uninfected epithelial cells9). Higher levels of RANTES are present in the respiratory secretions of RSV-infected children than in those of uninfected children10). In agreement with these findings, our study has demonstrated that the secretion of RANTES during RSV infection is increased in a murine model of asthma, thereby further confirming that RSV infection stimulates the synthesis of RANTES. Our finding t hat the increased secretion of RANTES following RSV-infection was also observed in PBS mice, and that there was no significant difference in RANTES levels between the PBS mice and our murine model of asthma (D. farinae mice), also agrees with previous results7).
Although previous studies have examined the synthesis of RANTES in bronchitis or bronchiolitis caused by RSV or other respiratory viruses, few studies have examined the level of RANTES during acute asthma attacks in either humans or animal models. Our study therefore examined RANTES expression in BAL fluid obtained from a murine model of acute asthma attack. Our finding that RSV infection induces RANTES production validates the results of a study reported by Matsuse et al.7); however, this increase was observed to occur regardless of asthma status, as there was no statistical difference in the levels of RANTES produced by our murine model of asthma compared with PBS-inoculated control mice. This result means RSV infection did not induce further increase of RANTES expression in this mouse model of asthma against PBS mice.
The cytokine IFN-γ is involved in the immune response to intracellular antigens, including viruses, encountered by cells. A previous study has reported that IFN-γ expression is increased in RSV-infected mice8) ; however, no significant difference was observed in our study. This discrepancy may be due to differences in the duration of RSV infection or in the measurement area.
RSV infection did not show any further increase of airway responsiveness in the murine model of asthma. This result differs with other study11). This may be due to the discrepancy in the time at which Penh values were measured following RSV infection between two studies.
The presence of eosinophils in the airway is a representative marker of asthma. Proteins produced by these cells mediate airway inflammation and are highly cytotoxic, causing the destruction of bronchial epithelial cells12) as well as inducing airway hyperresponsiveness. Neutrophils secrete IL-6, IL-8, and tumor necrosis factor-α and thus influence acute asthma attacks. In our study, we found that increased numbers of inflammatory cells, primarily eosinophils, were present in our murine model of asthma. Although increased numbers of inflammatory cells were also observed in the RSV-infected mice, the cells most increased by infection were the neutrophils. This is in agreement with the results of numerous previous studies.
Infection of our murine model of asthma with RSV decreased the number of eosinophils compared with the PBS mice, while no statistically significant difference in the number of lymphocytes was detected. This observation agrees with the results of 1 previous study 13). However, other groups have reported that there is an increase in the levels of eosinophils when mice are infected with RSV8,11). In our opinion, these discrepancies may be caused by differences in the individual mice used in these studies as well as in the length of time over which the experiments were carried out.
There was no accordance between the level of RANTES and the number of eosinophils in BAL fluid. It could be explained by the understanding that not only RANTES but also other factors can affect the number of eosinophils in the airway.
Our finding that the number of neutrophils present in the BAL fluid obtained from our murine model of asthma increased following RSV infection confirms the results of previous studies14). This result might mean that neutrophils are more important than eosinophils for the acute exacerbation of asthma by RSV infection. In contrast, we found that there was a reduction in the number of macrophages when our murine model of asthma was infected with RSV. Although this disagrees with other previous studies, which have found no statistically significant difference in macrophage numbers following RSV infection, this may again be due to a difference in measuring times.
Although the present study was limited in that IFN-γ expression and Penh were measured within one day of the introduction of RSV, it does provide insight into the events that occur shortly after RSV infection. This difference in timing may explain the discrepancies between our work and that of others, as many studies allow more time between RSV infection and subsequent measurements. However, we feel that since differences in RANTES and IFN-γ expression levels as well as Penh values and the cell composition of BAL fluid were observed within a day of infection, that this work provides worthwhile information. Perhaps most significantly, this study has also demonstrated that the level of RANTES synthesized in a D. farinae-sensitized murine model of asthma exacerbated by RSV infection is no different than that observed in PBS mice infected with RSV.
Acute asthma attack is a major cause of death in asthma patients. As the most significant cause of these attacks is viral infection of the respiratory tract, and RSV is one of the major causes of viral respiratory tract infections, determining the pathogenesis of RSV infection is an important undertaking. Our study has partially characterized the role of RANTES in acute asthma attack and has demonstrated that the level of RANTES produced following RSV infection was not increased to a greater extent in a murine model of asthma than in PBS mice. Further studies on the role of RANTES in acute asthma attack, as well as others examining the involvement of viruses other than RSV and other causes of acute exacerbation as well as the impact of the timing of acute asthma attack, may contribute to the prevention, diagnosis, and treatment of asthma in patients.
Acknowledgement
This research was supported by Seoul St. Mary's Clinical Medicine Research Program year of 2009 through the Catholic University of Korea.