Outcome of allogeneic hematopoietic stem cell transplantation for childhood acute lymphoblastic leukemia in second complete remission: a single institution study
Article information
Abstract
Purpose
The survival rate for childhood acute lymphoblastic leukemia (ALL) has improved significantly. However, overall prognosis for the 20 to 25% of patients who relapse is poor, and allogeneic hematopoietic stem cell transplantation (HSCT) offers the best chance for cure. In this study, we identified significant prognostic variables by analyzing the outcomes of allogeneic HSCT in ALL patients in second complete remission (CR).
Methods
Fifty-three ALL patients (42 men, 79%) who received HSCT in second CR from August 1991 to February 2009 were included (26 sibling donor HSCTs, 49%; 42 bone marrow transplantations, 79%). Study endpoints included cumulative incidence of acute and chronic graft-versus-host disease (GVHD), relapse, 1-year transplant-related mortality (TRM), disease-free survival (DFS), and overall survival (OS).
Results
Cumulative incidences of acute GVHD (grade 2 or above) and chronic GVHD were 45.3% and 28.5%, respectively. The estimated 5-year DFS and OS for the cohort was 45.2±6.8% and 48.3±7%, respectively. Only donor type, i.e., sibling versus unrelated, showed significant correlation with DFS in multivariate analysis (P=0.010). The rates of relapse and 1 year TRM were 28.9±6.4% and 26.4±6.1%, respectively, and unrelated donor HSCT (P=0.002) and HLA mismatch (P=0.022) were significantly correlated with increased TRM in univariate analysis.
Conclusion
In this single institution study spanning more than 17 years, sibling donor HSCT was the only factor predicting a favorable result in multivariate analysis, possibly due to increased TRM resulting from unrelated donor HSCT.
Introduction
Despite major improvements in the overall survival of children with acute lymphoblastic leukemia (ALL), for the 20 to 25% of patients who relapse, the prognosis remains poor1). Notwithstanding its potential complications, allogeneic hematopoietic stem cell transplantation (HSCT), preferably from a matched sibling donor (MSD), is known to result in better outcome rather than chemotherapy only in second complete remission (CR)2-4). With improvements in human leukocyte antigen (HLA) typing and supportive care of transplant-related complications, recent evidence suggests similar outcomes when comparing the results of MSD HSCT and matched unrelated (MUD) HSCT5-7). However, most of the patients undergoing transplantation will fail to be cured of their disease, emphasizing the need to identify important prognostic factors in children who receive HSCT, as well as novel therapeutic modalities.
Previous analyses indicate that the duration of first remission has overall prognostic significance for children with ALL who receive allogeneic HSCT after first relapse6,7). Other important factors include the conditioning regimen utilized as well as the disease status at the time of transplantation8,9). Efforts to identify other significant prognostic variables are key to maximizing the efficacy of allogeneic HSCT in childhood ALL, and improve upon its current shortcomings.
In this study, we retrospectively reviewed the results of allogeneic HSCT undertaken in children with ALL in second CR, during a period spanning more than 17 years at a single institution, with the aim of summarizing overall therapeutic outcome for these children, identifying major prognostic variables, and comparing our institution results for MSD HSCT and MUD HSCT.
Materials and methods
1. Patient cohort
1) Pre-transplantation
Patients diagnosed with ALL, who received allogeneic HSCT in second CR from August, 1991 to February, 2009 at the Department of Pediatrics, The Catholic University of Korea, were included. Recipients of cord blood transplantation or familial donor transplantation with 1 antigen or more mismatches were excluded from the study group. Overall, 53 patients (42 male, 79%) formed the study cohort (Table 1). Median age at diagnosis was 6.5 years (range, 0.3 to 15 years). Risk group classification before 2001 derived from National Cancer Institute criteria, and then, subsequent to 2001, was based on previously published institutional criteria10). Twenty patients (38%) were classified as low or standard risk ALL, and 33 (62%) were classified as high or very high risk ALL. Of 41 patients with available cytogenetic studies at diagnosis, 8 had high risk features including 4 patients with BCR-ABL1, 3 with E2A-PBX1, and 1 patient with MLL gene rearrangement.
All patients in the study cohort were diagnosed with first bone marrow relapse at a median of 32.6 months since achieving CR (range 1.1 to 118) (Table 2). For the purposes of risk stratification according to duration of first CR, patients were categorized into early relapse (<12 months since CR, n=11, 21%), intermediate relapse (≥12 months and <36 months since CR, n=18, 34%), and late relapse groups (≥36 months since CR, n=24, 45%)7).
2) Transplantation
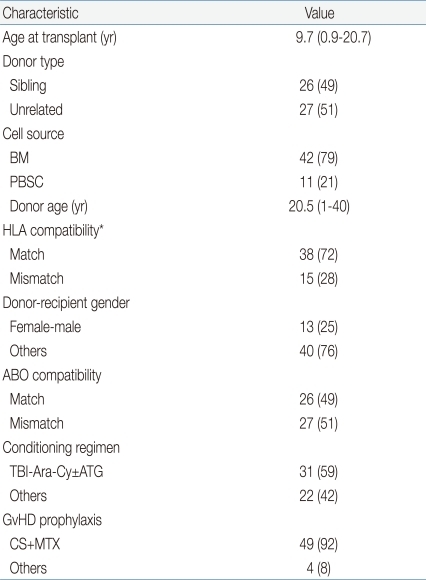
All patients underwent allogeneic HSCT after having achieved second CR (Table 3). Median age at transplantation was 9.7 years (range, 0.9 to 20.7 years). Similar numbers of sibling donor and unrelated donor transplantations were included (26 sibling, 49%; 27 unrelated HSCTs, 51%), but with regards to cell source, a far greater number of bone marrow transplantations (BMT) were included than granulocyte-colony stimulating factor mobilized peripheral blood stem cell transplantations (PBSCT) (42 BMTs, 79%; 11 PBSCTs, 21%).
As the study covered HSCTs undertaken during a lengthy period in time, HLA typing for the study cohort was also done with varying methodologies. Different modes of HLA typing posed a problem concerning patient classification according to HLA compatibility. A strict definition of "HLA-match" as an 8 of 8 allele complete match of high resolution-typed HLA, as is done currently in our institution, would classify many patients who underwent HSCT with a serologically matched donor, previous to implementation of high resolution typing, as having received "HLA-mismatched" transplantations. In order to resolve this issue, all patients were categorized according to time-appropriate HLA methodology; that is, an "HLA-match" was defined as a complete match of HLA tested between donor and recipient at that point in time, be the method serologic or molecular.
All but 2 patients received a total body irradiation (TBI)-based conditioning regimen, with TBI-cytarabine-cyclophosphamide±antithymocyte globulin (ATG) being the most commonly utilized regimen (n=31, 59%), followed by TBI-cyclophosphamide (n=15, 28%). Regimens without TBI included 1 busulfan-based and 1 carmustine-based conditioning each. Graft-versus-host disease (GvHD) prophylaxis consisted of cyclosporine and mini dose methotrexate (MTX) for 49 patients (92%), with the remaining patients receiving a combination of cyclosporine and mycophenolate mofetil±MTX.
2. Study endpoints
Major study endpoints included engraftment, incidence of acute GvHD (grades II to IV) and chronic GvHD, disease-free survival (DFS) and overall survival (OS). Neutrophil engraftment was defined as the first of 3 consecutive days in which the absolute neutrophil count (ANC) was >1,000/mm3, and platelet engraftment was defined as the first of 3 consecutive days in which the platelet count was >50,000/mm3 with no platelet transfusions in the past seven days. Acute and chronic GvHD were classified according to previously established criteria11,12). DFS was defined as the time from HSCT till relapse or death, whichever came first. OS was defined as time from HSCT till death from any cause. Relapse rate and rate of 1-year transplant-related mortality (TRM) were also evaluated.
3. Statistical analyses
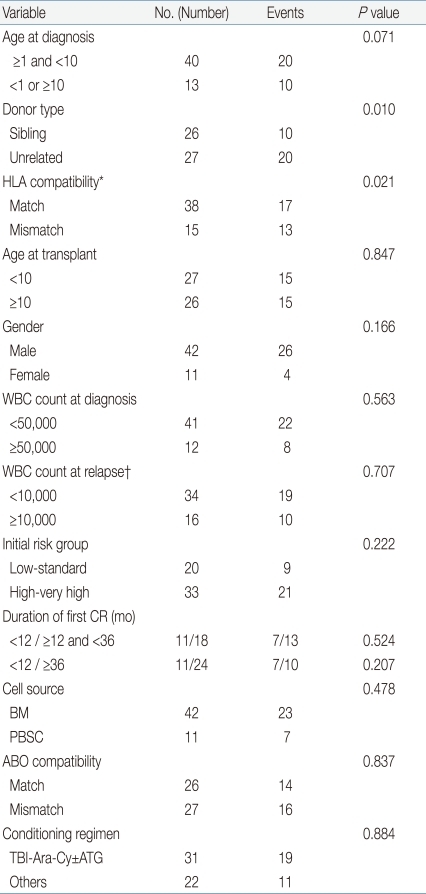
Prognostic factors for DFS were evaluated using Cox proportional hazards regression model. The following variables were analyzed: age at initial diagnosis, age at transplant, patient gender, white blood cell (WBC) count at diagnosis, WBC count at relapse, initial risk group, duration of first CR, donor type, cell source, HLA compatibility, ABO compatibility, and conditioning regimen. Variables with a P value of <0.1 on univariate study were entered in a multivariate model using the backward stepwise selection method. Incidence of acute and chronic GvHD, relapse and TRM rates were calculated using a cumulative incidence function with consideration of competing risks. Variables studied for DFS were also evaluated for contributing to acute and chronic GvHD, relapse rate and TRM rate using Gray's test. Again, factors with a P value of <0.1 were analyzed in a multivariate manner using Fine and Gray's proportional hazards model. DFS and OS were calculated with the Kaplan-Meier method and comparisons done with the log rank test. P values<0.05 were considered statistically significant. Statistical work was done on SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) and R package, ver. 2.10.1 (available from: http://cran.r-project.org).
Results
1. Engraftment data
Median infused cell counts were as follows: TNC 2.6×108/kg (range, 0.7 to 42.1×108/kg), MNC 1.2×108/kg (range, 0.5 to 35.7×108/kg), CD34+ 5.3×106/kg (range, 0.6 to 81.4×106/kg), CD3+ 5.2×107/kg (range, 0.5 to 518×107/kg). All patients showed neutrophil engraftment at a median of 15 days (range, 8 to 31 days), while only 38 patients (72%) showed full recovery of platelet count to greater than 50,000/mm3 at a median of 26 days (range, 10 to 178 days) post-transplantation.
2. Incidence of acute and chronic GvHD
Acute GvHD of grade II or above was diagnosed in 24 patients at a median of 15 days post-transplantation (range, 5 to 81) for a cumulative incidence of 45.3±6.9%. Eighteen patients (34%) were diagnosed with grade II acute GvHD, and 3 patients each (6%) were diagnosed with grades III and IV GvHD. All 3 patients with grade IV GvHD died. Important risk factors for acute GvHD included age at transplant (P=0.090), donor type (P<0.001), and HLA compatibility (P=0.002), with only HLA compatibility having a significant effect on multivariate study (hazard ratio [HR], 2.41; 95% confidence interval [CI], 1.14 to 5.08; P=0.021).
Chronic GvHD was diagnosed in 16 patients (mild 5, 9%; moderate 10, 19%; severe 1, 2%) for a cumulative incidence of 28.5±6.3%. Median time at chronic GvHD diagnosis was 5 months after transplantation (range, 2.7 to 17.2). No one factor had a significant impact on chronic GvHD incidence.
3. Analysis of DFS
The estimated 5-year DFS for the entire cohort was 45.2±6.8%. On analyzing for prognostic factors for DFS, age at diagnosis (P=0.071), donor type (P=0.010), and HLA compatibility (0.021) proved to be important variables (Table 4). However, on multivariate study, only donor type had prognostic significance (HR, 2.75; 95% CI, 1.28 to 5.93; P=0.010), with superior DFS in recipients from sibling donors compared to unrelated donors. Patients with a long duration of first CR before HSCT did not have a survival advantage compared with patients who relapsed early.
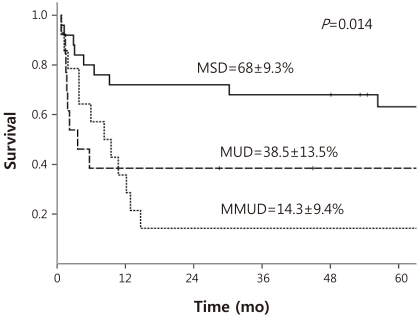
DFS curves were redrawn in order to evaluate for potential comparable outcomes of MSD and MUD HSCT (Fig. 1). Five-year estimated DFS for MSD HSCT, MUD HSCT, and mismatched unrelated donor (MMUD) HSCT were as follows: 68±9.3%, 38.5±13.5%, 14.3±9.4% respectively. Log-rank comparison of MSD and MUD HSCT showed a value bordering on statistically significant difference (P=0.051), while a comparison of MSD HSCT and MMUD HSCT showed clear differences in DFS (P=0.002).
4. Relapse and TRM incidence
Overall, 15 patients relapsed resulting in an estimated 5-year rate of disease relapse of 28.9±6.4%. All patients showed bone marrow (BM) relapse except for 1 patient who was diagnosed with isolated testicular relapse 9 months post-transplantation. Analysis was done to determine factors significant for relapse using the variables utilized for study of DFS. Initial WBC count at diagnosis had prognostic relevance bordering on significance, with patients presenting with an initial WBC count ≥50,000/mm3 more likely to relapse than those with lower counts (P=0.082). However, no one variable had a definite significant impact on rate of relapse, as determined by univariate analysis. All patients with BM relapse, except 2 who were lost to follow-up within 1 year of relapse, died. The patient with isolated testicular relapse underwent localized radiotherapy and chemotherapy and has survived for 89 months post-HSCT at last follow-up.
Another 15 patients died during the initial year post-transplantation due to causes other than relapse for a 1-year cumulative TRM incidence of 26.4±6.1%. Although univariate study of prognostic factors for TRM revealed donor type (P=0.002) and HLA compatibility (P=0.022) to be significant variables, none proved to maintain significance on multivariate study. Causes of TRM were as follows: pneumonia and acute respiratory distress syndrome 5, acute GvHD 3, thrombotic microangiopathy 2, pulmonary hemorrhage 2, venoocclusive disease 1, sepsis 1, and acute renal failure 1. Nearly all non-relapse deaths occurred during the first year post-transplantation, with only 1 patient dying of chronic GvHD beyond this period.
5. Analysis of overall survival
The estimated 5-year OS for the entire cohort was 48.3±7%, showing a rate similar to the DFS. Reanalysis according to donor type with consideration of HLA compatibility resulted in the following OS rates: 75±8.9% for MSD, 38.5±13.5% for MUD, and 14.3±9.4% for MMUD. Significant differences were noted when comparing the OS for MSD to that for either MUD (P=0.018) or MMUD (P<0.001).
Discussion
In this study, we report the outcomes of allogeneic HSCT undertaken in children with ALL in second CR at a single institution during a lengthy period spanning more than 17 years. As far as we know, this is the first such report to derive from a Korean pediatric population.
The overall 45.3±6.9% incidence of grade II to IV acute GvHD was considerably higher than reported rates in similar studies6,7). The lack of routine in vivo T cell depletion with the use of ATG in the conditioning regimen for unrelated HSCTs done in the early period of the study may have had a role in increasing acute GvHD incidence. Cumulative incidence of 28.5±6.3% for chronic GvHD was similar to the 22 to 27% reported from large cohort studies comprising both matched sibling and alternative donor HSCTs13,14).
Overall, less than half the patients of the cohort survived disease-free. Also of note, the DFS and OS were similar emphasizing once more the extremely poor prognosis once a patient relapsed after HSCT. Excluding patients lost to follow-up, only 1 patient with isolated testicular relapse survived after relapse. In comparison, the DFS of 45.2% for the overall cohort is similar to previous reports based on HSCTs from heterogenous donor types7,15), but is considerably lower than the 67.1% 3-year DFS reported by one study6) which also included patients transplanted in first CR. Sub-analysis according to donor type lends some evidence for this discrepancy; although the estimated DFS of 68% for MSD HSCT was superior or comparable to previous results, the 38.5% DFS for MUD HSCT was much lower6,7). Hence, unlike these reports which conclude on similar results for MSD and alternative donor HSCT, there was at least a trend towards a significant difference when comparing MSD and MUD HSCT (P=0.051).
Akin to the survival analyses, the regression modeling revealed that donor type, sibling versus unrelated donor, was the only significant prognostic factor for DFS. As all sibling donors were fully HLA-matched except for 1 donor who showed 1 allele mismatch with a sibling patient, how much of the prognostic significance of a sibling donor pertains to HLA compatibility, and how much is based on the potential benefits of a sibling donor beyond standard HLA typing would require further comparative study of a cohort consisting of MSD and high resolution-typed MUD HSCTs only. Our study also revealed the lack of a survival benefit for patients who underwent HSCT after a lengthy period of first remission compared to those who relapsed early on during treatment. This is an important finding considering that nearly half the patients (45%) in our cohort relapsed after at least 3 years of CR status. Previous studies indicate either a survival advantage for late relapse patients7), or are inconclusive as to whether HSCT improves upon the approximately 50% survival rate based on chemotherapy only16), and our results underscore the important question of whether patients with late relapse may benefit from HSCT.
No one factor had a significant impact on the 29% cumulative relapse rate, although a previous study has shown that HSCT from an MUD leads to a lower rate of relapse17). With regards to 1-year TRM, both donor type and HLA compatibility were significant factors individually, but none proved to be important on multivariate study, possibly due to data multicollinearity between the 2 variables. However, it is important to note that donor type, which was the only significant factor for DFS, was also important in terms of 1-year TRM.
We draw attention to the limitations of our study. First, as mentioned previously, the lengthy span of time during which the HSCTs took place led to heterogeneity in HLA typing methods, from low to high resolution typing, posing problems for patient classification according to HLA compatibility. Decision as to whether a patient was matched or mismatched was, therefore, based on time-appropriate HLA methodology. Survival analyses with subclassification of MUD HSCT according to HLA typing method, for example high resolution HLA-MUD versus low resolution HLA-MUD, would have given some insight into the importance of typing accuracy. However, the number of patients in each subgroup then proved to be too few to allow for accurate analysis. Lack of specific cytogenetic data for each patient is also conspicuous and was due to many patients transplanted in the early period not having accurate cytogenetic characterization. Cytogenetic studies and minimal residual disease monitoring pre-transplant based on such studies have since gained importance18), and should be incorporated into future studies of more recent cohorts.
Also, the potential efficacy of HSCT, especially in the MSD setting as shown by our results, should be tempered by the substantial risk for late effects in children who undergo HSCT. The number of physical organs and functions that are susceptible to delayed complications of transplantation are considerable, with 1 recent report indicating a 78% incidence of late effects amongst HSCT treated childhood cancer survivors19).
In conclusion then, with less than half the overall cohort surviving disease free, donor type had a significant impact on survival, possibly due to increased TRM resulting from unrelated donors. Survival rates for MSD HSCTs were comparable to previous studies, but those of unrelated HSCTs tended to be lower. Recognizing that many of the patients in our study underwent HSCT more than a decade ago, and understanding the major improvements in supportive care and HLA typing methods that have taken place since then, similar studies with more recently treated cohorts should be undertaken to conclude upon possible improvements in unrelated donor HSCT results. With more than half of childhood ALL patients in second CR failing to be cured by HSCT, both means of optimizing HSCT treatment through recognition of new prognostic factors and research into other novel therapeutic options remain necessary.