A case of acute necrotizing encephalopathy associated with parainfluenza virus infection
Article information
Abstract
Acute necrotizing encephalopathy (ANE) may be suspected when a young child presents with abrupt onset of altered mental status, seizures, or both. Definitive clinical diagnosis is based on magnetic resonance imaging (MRI) results. ANE is associated with influenza virus infections. Preliminary data suggests that up to 25% of ANE patients die, and up to 25% of ANE survivors develop substantial neurologic sequelae. Here, we describe a case of a comatose 22-month-old girl who was admitted to our hospital because of febrile illness and seizures. On day 13 of her illness, she died from ANE associated with infection from parainfluenza virus. Brain MRI results indicated diffuse bilateral symmetric signal changes in both basal ganglia, thalami, periventricular white matter, pons, and cerebral white matter, as well as generalized swelling of the brain.
Introduction
Acute necrotizing encephalopathy (ANE) is a severe parainfectious disorder with specific neuroimaging findings. ANE is a rare disease firstly described by Mizuguchi et al.1), which affects young children in Far East countries, mainly Japan and Taiwan2), and recently also in North America and Europe3-5). The clinical characteristics of this disorder are encephalopathy soon after a febrile illness, elevation of serum aminotransferase1,2), diffuse, bilateral, symmetric high intensity signal on T2-weighted brain magnetic resonance imaging (MRI), edema, and apoptosis and necrosis of neurons without inflammation evident in brain microscopy. A previous viral infection is needed for definite diagnosis, and neuroimaging findings are needed to differentiate ANE from other para- or post-infectious encephalopathies and encephalitis.
ANE appears to be most common in Asian children, but there is a previous report of this disorder in a Caucasian child3). In the present case report, we describe a Korean child with ANE associated with parainfluenza virus infection.
Case report
A 22-month-old girl was admitted to our hospital with a three-day history of cough and rhinorrhea, a one-day history of fever, and an episode of generalized tonic clonic seizure with fever that lasted around 10 to 15 minute at 15 hours before admission.
Upon arrival at the emergency center, her pulse rate was 140/min, blood pressure was 115/74 mmHg, respiratory rate was 28 breaths/min, and body temperature was 40.2℃. She had no spontaneous movement, no response to painful stimuli, no pupillary reflex, and no corneal reflex. Doll's eye movement was absent. Her pupils were 1 mm in diameter and nonreactive to light. Deep tendon reflexes were absent.
Approximately 1 hour after arrival she had a second generalized tonic clonic seizure with fever that lasted 10 seconds, and was treated with 0.3 mg/kg of intravenous diazepam. She had dark brown-colored hematemesis, so we performed L-tube irrigation with normal saline. A computed tomography (CT) scan indicated diffuse symmetric low density in the basal ganglia, thalami, and pons with brain swelling (Fig. 1). Because of brain swelling, unstable vital sign, and thrombocytopenia, we decided to defer lumbar puncture and after all we did not make it. She was admitted to the intensive care unit.
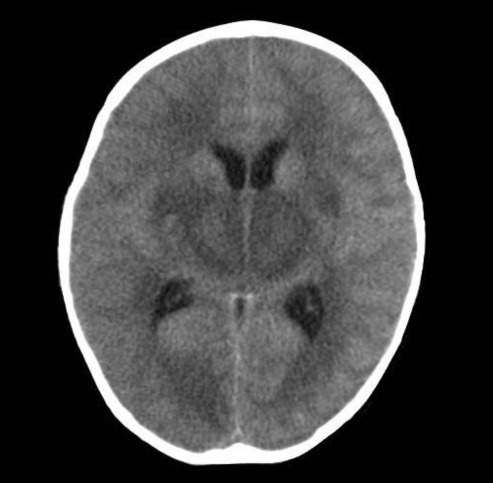
Computed tomography scan on admission shows diffuse symmetric low density in both basal ganglia, thalami, and pons with brain swelling.
Her platelet count was 32,000/µL, white blood cell was 6,240/µL, aspartate aminotransferase was 4,579 U/L, alanine aminotransferase was 2,787 U/L, ammonia was 38 mmol/L and C-reactive protein was 1.0 mg/dL. Coagulation testing for prothrombin, partial thromboplastin and thrombin times, fibrin degradation products, and D-dimer were elevated. Blood urea nitrogen was 30 mg/dL and serum creatinine was 1.3 mg/dL. Creatine kinase-MB, myoglobin, and troponin-T were elevated. Respiratory virus polymerase chain reactions for influenza A and B, respiratory syncytial virus, adenovirus, metapneumovirus, and rhinovirus were negative, but the test for parainfluenza virus was positive on a nasal wash specimen. Serological tests for hepatitis viruses, mycoplasma, herpes virus, Japanese encephalitis virus, and salmonella were all negative.
Electroencephalography indicated extremely low voltage and featureless background rhythms (Fig. 2). Brain MRI performed on the day of hospitalization indicated diffuse bilateral symmetric signal changes of both basal ganglia, thalami, periventricular white matter, pons, and cerebral white matter, as well as generalized swelling of the brain. The involved areas were diffusely hypointense on T1W images (Fig. 3B) and hyperintense on T2W images (Fig. 3A). We diagnosed the patient with ANE based on the clinical symptoms and MRI findings.
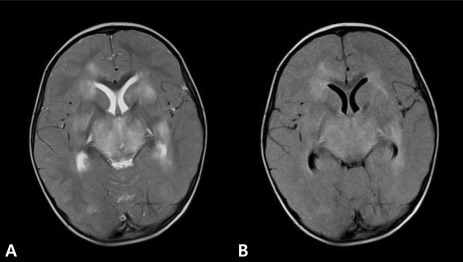
Magnetic resonance imaging shows diffuse bilateral symmetric signal changes in both basal ganglia, thalami, periventricular white matter, pons, and cerebellar white matter, with associated brain swelling.
The patient was intubated and mechanically ventilated because of shallow breathing and profound alteration of sensorium on the day of hospitalization and she was treated with ceftriaxone, vancomycin, acyclovir, intravenous immunoglobulin, and methylprednisolone. A chest X-ray was normal at admission, but indicated right perihilar infiltration on the 5th day of admission. Her consciousness had no interval change, and blood pressure, heart rate, and urine output decreased on day 13. The 14th day of admission, she died due to multiple organ failure.
Discussion
In 1995, Mizuguchi et al.1) first suggested that ANE was a novel disease entity and proposed the following diagnostic criteria: 1) acute encephalopathy following viral febrile disease with rapid deterioration in consciousness and/or convulsion; 2) increased protein in cerebrospinal fluid (CSF) without CSF pleocytosis; 3) CT or MRI findings indicating symmetric, multifocal brain lesions involving bilateral thalami, cerebral periventricular white matter, internal capsule, putamen, upper brain stem tegmentum, and cerebellar medulla without involvement of other central nervous system regions; 4) variable amount of serum aminotransferase elevation, but no hyperammonemia; 5) exclusion of other similar diseases such as overwhelming bacterial and viral infections, fulminant hepatitis, Reye syndrome, Leigh encephalopathy and related mitochondrial cytopathies, acute disseminated encephalomyelitis and other types of encephalitis, vasculitis, and cerebral infarction1,2).
The etiology of ANE is unknown2). Our patient had evidence of parainfluenza virus infection but this appears to be only rarely associated with ANE. Parainfluenza virus is a common pathogen in young children, which usually presents as a respiratory illness and less frequently as an undifferentiated febrile illness with vomiting, diarrhea, hypotonia, irritability, and meningismus6,7). Neurologic complications such as Guillain-Barré syndrome, Reye syndrome8,9), and acute disseminated encephalomyelitis10) may occur.
ANE is associated with significant morbidity and mortality, and survivors often experience at least short-term neurologic sequelae1). There is no specific therapy for ANE. Some patients improve if corticosteroid is administered during the acute stage11,12), but the clinical usefulness of corticosteroid treatment has not been definitively established. Okumura et al.13) concluded that administration of steroid within 24 hours of the onset of ANE was associated with better outcome of children with ANE who did not have brainstem lesions. Methylprednisolone was given to our patient, although she had brainstem lesions and diagnosis was made after 24 hours. Other research has suggested the efficacy of hypothermia and early anticytokine therapy in some cases of ANE14).
In summary, we describe a pediatric patient infected with parainfluenza virus who presented with ANE and progressed to brain death. Parainfluenza virus is generally considered a benign illness in healthy children, but our report of an association with ANE indicates a high degree of clinical suspicion for a child who presents with acute mental change, febrile seizure, and neurologic symptoms in the presence of parainfluenza infection.
