Effects of coagulation factor concentrate prophylaxis in moderate and severe hemophilia A patients at a single hemophilia center in Korea
Article information
Abstract
Purpose
The aim of this study was to investigate prophylactic treatment effects in Korean patients with severe hemophilia A.
Methods
A prospective study of 32 severe hemophilia A patients was conducted with the approval of the Institutional Review Board at the Eulji University Hospital. Two patients received primary prophylaxis; whereas, the other 30 patients were divided into 2 groups-secondary prophylaxis (n=15) and on-demand (n=15)-on the basis of their consent for secondary prophylaxis. A 20-25 IU/kg dose of factor VIII concentrate was administered to the primary and secondary prophylaxis group patients every 3 days for 1 year. The prophylactic effect was evaluated by observing changes in the Pettersson scores, annual number of total and joint bleeds, and factor VIII consumption for 1 year.
Results
No moderate or severe bleeding was observed, and the Pettersson scores remained unchanged during the prophylaxis period in the patients who received primary prophylactic treatment. After the treatment was changed from on-demand to secondary prophylaxis, the annual number of total and joint bleeds in the secondary prophylaxis group decreased by 64.4%±13.0% and 70.0%±15.2%, respectively. The average increase in Pettersson scores within 1 year was 0.5±0.8 and 1.3±1.1 in the secondary prophylaxis and on-demand groups, respectively. Prophylactic effects were also observed in patients >17 years who had nearly the same initial Pettersson scores.
Conclusion
Intermediate-dose prophylactic treatment may delay hemarthropathy progression and prevent its occurrence in Korean severe hemophilia A patients.
Introduction
The most common bleeding episodes in patients with hemophilia are intra-articular hemorrhages. Repeated hemarthrosis progressively damages the intra-articular cartilage, which eventually results in chronic hemophilic arthritis and disabilities of the involved joints1,2). Hemarthropathy progresses as the cumulative number of joint hemorrhages increases, irrespective of the strength of the on-demand treatment3). Fortunately, the current prophylactic treatment can completely prevent hemophilic arthropathy if it is started at an early age, and it can delay the progression of hemarthropathy by reducing the frequency of joint bleeds3-7).
Primary prophylaxis commenced at a very young age (generally before the age of 2 or 3 years) and before development of joint disease can be distinguished from prophylaxis started after the onset of some joint damage, generally at an older age (secondary prophylaxis). The initial idea of prophylactic treatment was based on the observation that patients with moderate hemophilia, which is defined as factor activity of 1-5%, experienced fewer joint bleeds and consequently less joint damage than patients with severe hemophilia, which is defined as factor activity less than 1%8). Based on this observation, prophylactic treatment was started in patients with severe hemophilia A by Nilsson et al.4) in order to change the phenotype of patients with severe hemophilia to a moderate one by regular injections of coagulation factor concentrate (CFC) to maintain the trough factor activity higher than 1%. This treatment has been applied widely to patients with severe hemophilia A since the early 1970s, because large amounts of CFCs were produced commercially after the discovery of cryoprecipitate by Judith Pool in 19649). The beneficial effects of prophylaxis have been reported in many papers since then3-7).
Based on these results, in 1995, the World Health Organization and World Federation of Hemophilia recommended prophylactic treatment as a first-line treatment for patients with severe hemophilia10). The standard prophylactic regimen is 20-25 IU/kg of FVIII CFC given every 48 hours for patients with severe hemophilia A and 25-40 IU/kg of FIX CFC given every 72 hours for patients with severe hemophilia B in order to maintain the trough factor activity higher than 1%. Several variations of this standard regimen, such as low- to intermediate-dose prophylaxis, are currently being given to patients with severe hemophilia, depending on the available CFC resources and venous accessibility11,12). However, there are no reports on the effects of prophylaxis in Korean patients with hemophilia because this treatment is not reimbursed by national health insurance. Hence, the present study was conducted to determine the effects of prophylaxis in Korean patients with severe hemophilia A.
Materials and methods
1. Patients
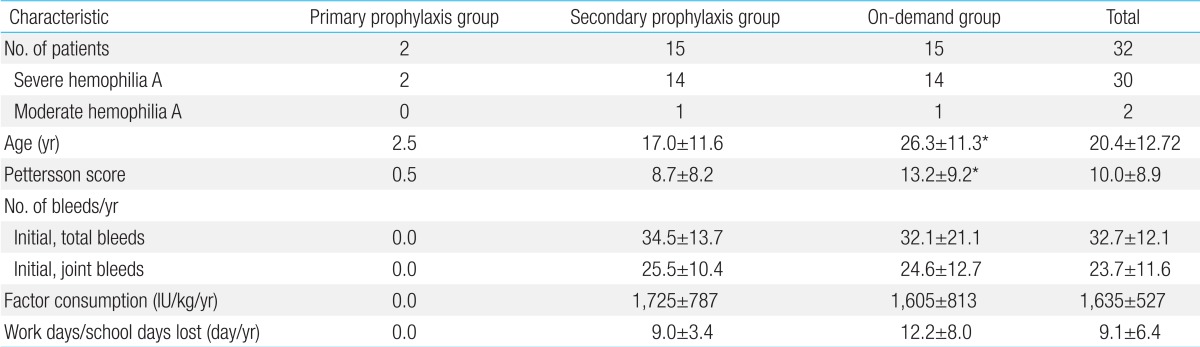
This study was prospectively conducted in 32 hemophilia A patients, including 2 newly diagnosed young children, who were registered at the Eulji University Hospital. All patients had severe hemophilia A, except for 2 patients with moderate hemophilia A, based on the basal FVIII level. However, patients with moderate hemophilia A showed clinical characteristics of the severe phenotype (≥1.0 joint or muscle bleedings/wk) (Table 1). All patients gave their consent for participation after receiving meticulous explanations regarding the study, and the Institutional Review Board at the Eulji University Hospital accepted this study. There was no history of inhibitors in the patients except for history of previous transient inhibitors in 2 patients. Only on-demand treatment had been given to the patients before the present study. When muscle and joint bleeding occurred, 1-3 doses of 20-25 IU/kg of plasmaderived or recombinant FVIII CFC had been delivered to the patients at home or in the clinics.
2. Methods
Primary prophylaxis was given to the 2 patients with newly diagnosed severe hemophilia A and basal factor activity less than 1%. Primary prophylaxis was started at the age of 2 and 3 years, before the occurrence of any episodes of joint bleeds and other life or limb-threatening bleeds since birth. A dose of 20-25 IU/kg of recombinant FVIII CFC was regularly delivered to these 2 patients every 3 days for 1 year. The other 30 patients, who had received only on-demand treatment, were divided into 2 groups based on their consent for secondary prophylaxis. Fifteen patients who did not give consent to receive secondary prophylaxis continued to receive on-demand treatment for 1 year, which was the same as the on-demand treatment given before the present study (on-demand group). The remaining 15 patients agreed to receive secondary prophylaxis (secondary prophylaxis group). The same plasma or recombinant FVIII CFC that had been used in the on-demand treatment of each patient before the present study were provided to the patients in the secondary prophylaxis group, and a dose of 20-25 IU/kg of FVIII CFC was regularly delivered to these patients every 3 days for 1 year. When breakthrough bleeds were observed, the same on-demand treatments were delivered to the patients in the secondary prophylaxis group.
The effects of prophylaxis in all patients in the secondary prophylaxis group were evaluated by observing changes in the Pettersson scores, annual number of bleeds and joint bleeds, annual factor consumption, and the number of work or school days lost before and after 1 year of the initiation of the present study. Simple radiographs of the knee, ankle, and elbow joints were obtained to assess the joint state by using Pettersson scoring13) in each individual just before and 1 year after the start of the present study. The annual number of bleeding episodes and data on FVIII CFC consumption were obtained by reviewing medical records and self-injection diaries. The number of work or school days lost was obtained through the questionnaires. The same data were also collected from patients in the on-demand group and were compared with that from patients in the secondary prophylaxis group. Inhibitors were measured every 6 months during the study period. Paired t test was used for comparisons, and P<0.05 was considered statistically significant.
Results
All 32 subjects completed this study. No patients experienced life-threatening bleeds or underwent surgeries during the 1 year study period. Inhibitors were not detected in the patients during the study period. The average age and the Pettersson score for all subjects were 20.4±12.72 years and 10.0±8.9, respectively. The average annual number of total and joint bleeds before the study period, for which coagulation factor supplements were required, was 32.7±12.1 and 23.7±11.6, respectively; the average annual FVIII CFC consumption was 1,635±527 IU/kg (Table 1).
1. Results of the primary prophylaxis
The Pettersson score before the start of prophylaxis was 0.0 in 1 patient in whom primary prophylaxis was started at 2 years of age. However, the Pettersson score was 1.0 in another patient in whom primary prophylaxis was started at 3 years of age, despite no clinical joint bleeds detected since birth (Table 1). No moderate or severe bleeding was observed during the prophylaxis period in these 2 patients. The Pettersson scores remained stable at 0.0 and 1.0, respectively, after 1 year of primary prophylaxis.
2. Results of the secondary prophylaxis
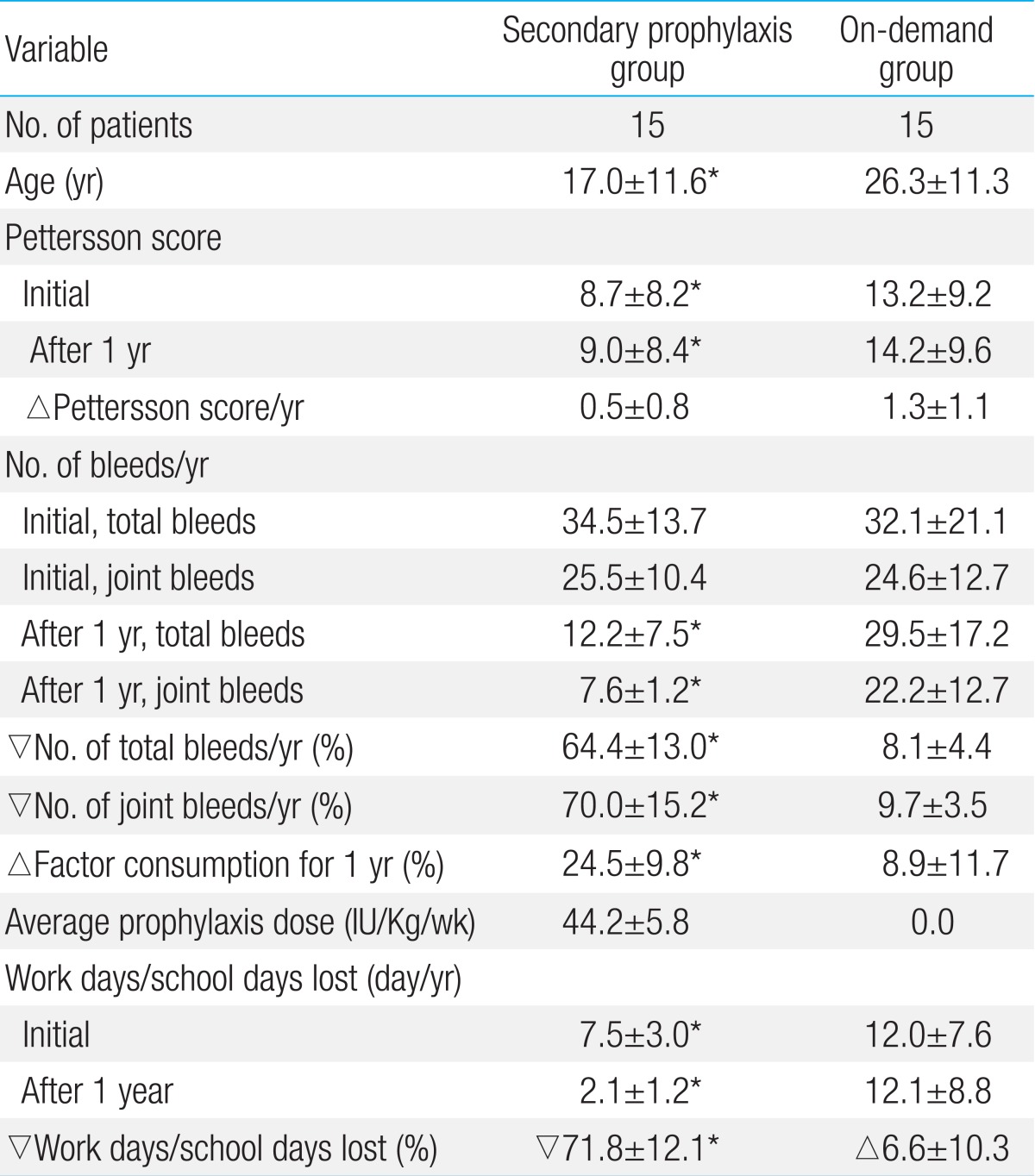
The average age of the patients in the secondary prophylaxis and on-demand groups was 17±11.6 years and 26.3±11.3 years, respectively, and the average Pettersson score was 8.7±8.2 and 13.2±9.2, respectively. The average number of total and joint bleeds and the average annual factor consumption before the study were not different between the 2 groups (Table 1). After the change in treatment from on-demand to secondary prophylaxis, the number of total and joint bleeds decreased by 64.4%±13.0% and 70.0%±15.2%, respectively, compared with the number of bleeds before the start of prophylaxis in the secondary prophylaxis group. In contrast, the number of total bleeds and joint bleeds decreased by 8.1%±4.4% and 9.7%±3.5%, respectively, in the ondemand group (Table 2). The average increase in the Pettersson score during the study period was 0.5±0.8 in the secondary prophylaxis group and 1.3±1.1 in the on-demand group. The average number of work days or school days lost was decreased by 71.8% in the secondary prophylaxis group. In contrast, the average number of work days or school days lost was increased by 6.6% in the ondemand group. The average annual factor consumption was increased by 24.5% in the secondary prophylaxis group and by 8.9% in the on-demand group. The average weekly prophylaxis dose was 44.2±5.8 IU/kg (Table 2).
3. Effects of secondary prophylaxis depending on the initial Pettersson scores
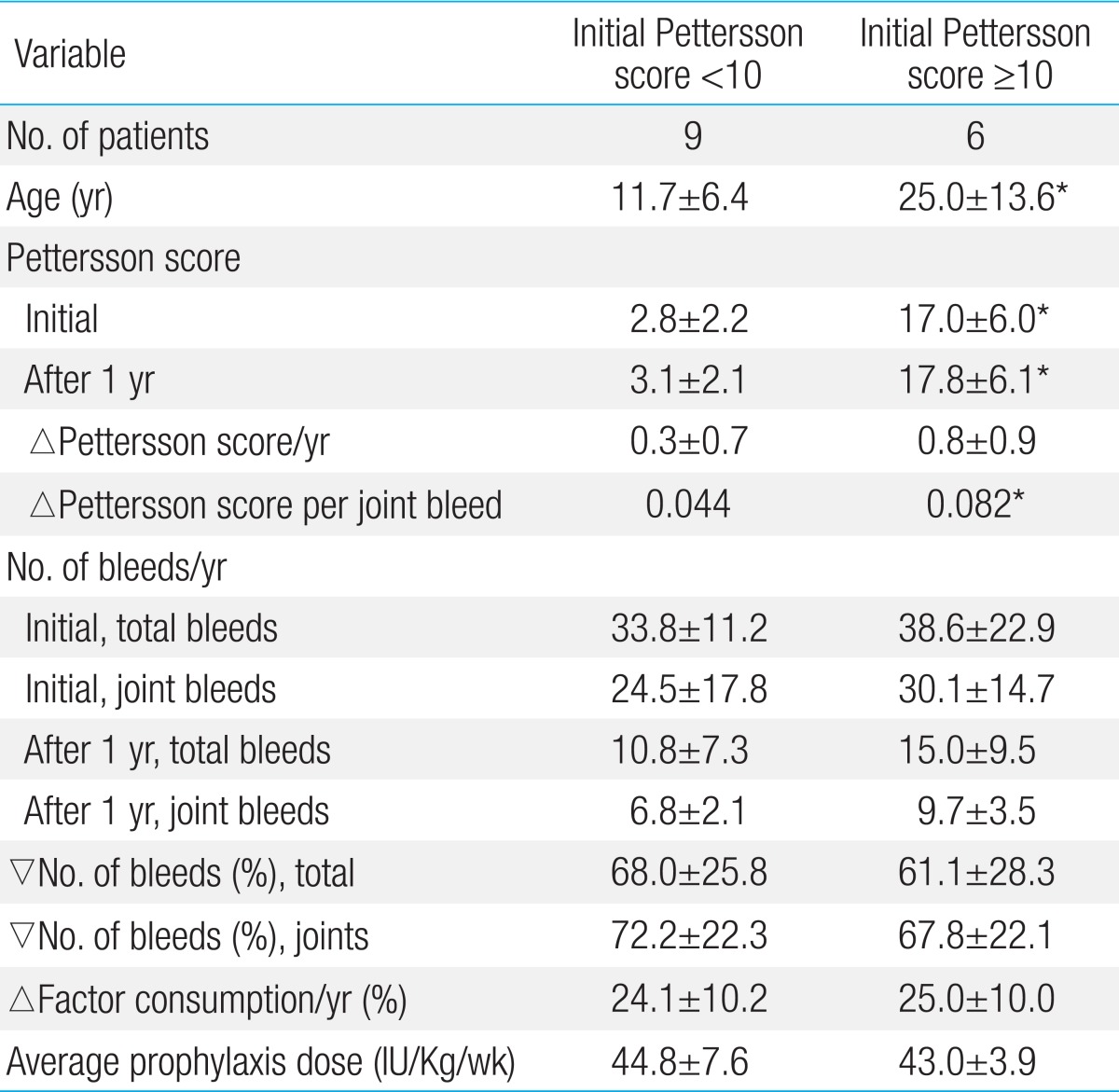
To understand the effects of the initial stages of hemarthropathies on the results of secondary prophylaxis, the patients receiving secondary prophylaxis were grouped according to the initial Pettersson scores. Patients with Pettersson scores less than 10.0 were in the Pettersson score<10 group (9 patients), and the patients with Pettersson scores more than 10.0 (6 patients) were in the Pettersson score≥10 group. When the data were compared between groups, changes in the number of total and joint bleeds, FVIII CFC consumption, and average prophylaxis dose during the study period were not different between the 2 groups (Table 3). However, the increase in the Pettersson score per joint bleed during the study period was different. Although it was not statistically significant, this difference was nearly twice of that in the Pettersson score<10 group (△0.044 Pettersson score per joint bleed in the Pettersson score<10 group vs. △0.082 Pettersson score per joint bleed in the Pettersson score≥10 group) (Table 3).
4. Effects of secondary prophylaxis in late adolescent and adult patients with severe hemophilia A
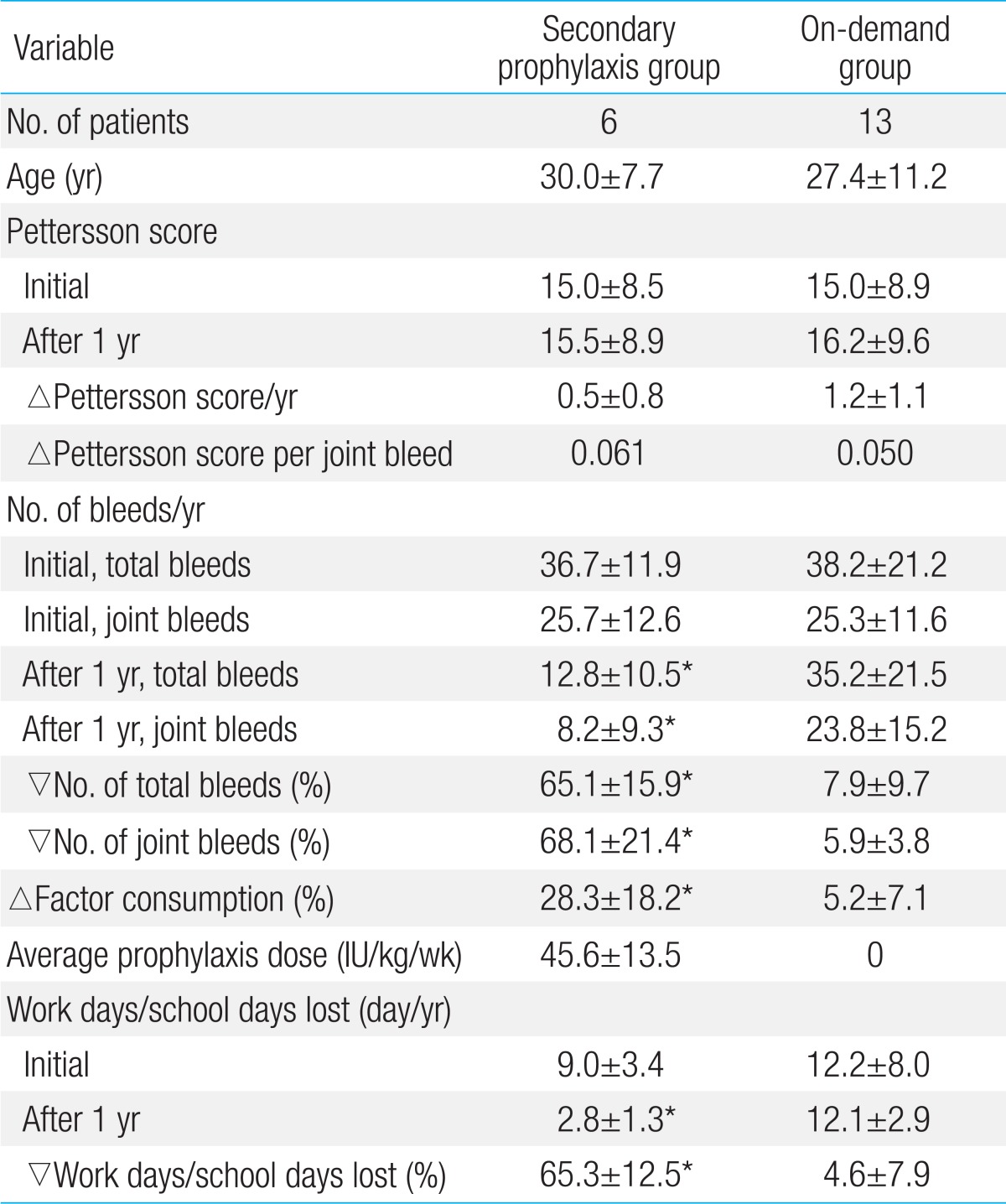
To understand the effects of secondary prophylaxis in late adolescent and adult patients, changes in the average Pettersson scores, annual total and joint bleeds, and factor consumption were compared between patients in the secondary prophylaxis group and patients in the on-demand group who were older than 17 years. There were 6 patients in the secondary prophylaxis group and 13 patients in the on-demand group. The average Pettersson scores, ages, annual number of total and joint bleeds, and factor consumption before the study were similar between the secondary prophylaxis and on-demand groups. The effects of prophylaxis were also obvious in these patients. After change in treatment from on-demand to secondary prophylaxis, the number of total and joint bleeds decreased by 65.1%±15.9% and 68.1%±21.4%, respectively, in the secondary prophylaxis group. In contrast, the number of total and joint bleeds decreased by only 7.9%±9.7% and 5.9%±3.8% in the on-demand group (Table 4). The Pettersson scores before the study were nearly the same in the patients in the secondary prophylaxis and on-demand groups (15.0±8.5 vs. 15.0±8.9) who were older than 17 years. Therefore, as expected, the increase in the Pettersson score per joint bleed (△0.061 in the secondary prophylaxis group vs. △0.050 in the on-demand group) during the study period was not different between the 2 groups. However, the increase in the average Pettersson score in the on-demand group during the study period was nearly twice the increase in the Pettersson score in the secondary prophylaxis group (△0.5 in the secondary prophylaxis group vs. △1.2 in the on-demand group) because joint bleeds were much more frequent in the on-demand group (Table 4). In addition, the average number of work days or school days lost was decreased by 65.3% in the secondary prophylaxis group compared with a decrease of 4.6% in the on-demand group. The average annual factor consumption was increased by 28.3%±18.2% in the secondary prophylaxis group and by 5.2%±7.1% in the on-demand group (Table 4).
Discussion
1. Effect of primary prophylaxis
Most patients with severe hemophilia experience their first clinical joint bleed at approximately 2 years of age, and the annual Pettersson score increases by 8% (1-16%) if prophylaxis is delayed14,15). Although the primary prophylactic treatment was given only for 1 year, the present study demonstrated the possibility of complete prevention of arthropathy in 1 patient in whom the initial Pettersson score was 0.0 and prophylactic treatment was started at 2 years of age. This effect may be due to the complete prevention of clinical joint bleeds because no moderate or severe bleeding was observed during the study period in the patient. Another possible explanation of this effect may be the difference in the clinical phenotypes in patients with severe hemophilia A. It is known that 10-15% of patients with severe hemophilia A rarely bleed spontaneously14,16). This difference in the phenotypes makes it difficult to decide the age at which primary prophylaxis should be started because this difference can be acknowledged only after the first clinical joint bleed. However, the start of primary prophylaxis after the first clinical joint bleed may be associated with a risk of developing progressive damage to the joint cartilage17).
Furthermore, the subclinical joint bleed may need to be considered when deciding on the start of primary prophylaxis. In this study, the Pettersson score was 1.0 before the start of primary prophylaxis in another patient, although clinical joint bleeds had not been detected since birth. This finding showed that subclinical joint bleeds can begin at a very young age before clinical bleeds are detected. Therefore, it may be ideally recommended that primary prophylaxis should be given to patients with severe hemophilia A as early as possible when the patients begin to walk, although there are no observed clinical joint bleeds in these patients. A progressive increase in the intensity of the prophylaxis may be a pragmatic method of primary prophylaxis when venous access is difficult and the difference in the phenotypes has been considered12,18).
2. Effects of secondary prophylaxis
Repeated hemarthrosis in patients with hemophilia progressively damages the intra-articular cartilage, which eventually results in chronic hemophilic arthritis and disability of the involved joint1,2), which are the main causes of low quality of life (QoL) in patients19). The cumulative number of joint bleeds can be reduced only by prophylactic treatment3). The objective of secondary prophylaxis is to delay the progression of arthropathy by reducing the number of joint bleeds, and this effect of secondary prophylaxis in Korean patients with severe hemophilia A was seen for the first time in this study. The frequency of all bleeds and joint bleeds was markedly decreased in the secondary prophylaxis group compared with that in the nonprophylaxis group. The increase in Pettersson scores was also less in the secondary prophylaxis group, although the consumption of FVIII CFC was higher in the secondary prophylaxis group.
However, the initial Pettersson scores were higher in the nonprophylaxis group in the present study. The rate of progression of arthropathy may be different, depending on the stages of arthropathies in patients. According to the longitudinal study by Fischer et al.20) on changes in the Pettersson score and the cumulative number of hemarthroses in patients with severe hemophilia, the Pettersson score increased slowly up to 20 years of age, reaching 10.0 with an approximate annual increase of 0.67; however, after that, the Pettersson score increased rapidly up to 30 years of age, reaching 25 with an annual increase of 1.5. This difference in the rate of progression of arthropathy was also observed in this study. The rate of increase in the Pettersson score per joint bleed was nearly twice of that in patients in the secondary prophylaxis group with an initial Pettersson score higher than 10.0 compared with the patients in the secondary prophylaxis group with an initial Pettersson score lower than 10.0, although the average number of joint bleeds during the study period was not different between the groups. Therefore, the effects of prophylaxis on hemarthropathy can be precisely evaluated when the effects of different treatments are compared between patients with similar stages of hemarthropathies.
The effects of prophylaxis in this study were also obvious when compared only in patients older than 17 years. The initial Pettersson scores in the secondary prophylaxis and on-demand groups were nearly the same, but the number of total bleeds and joint bleeds was markedly decreased in the secondary prophylaxis group after change of treatment from on-demand to secondary prophylaxis. As expected, the increase in the Pettersson score per joint bleed during the study period was not different between the 2 groups. However, the average increase in the Pettersson score in the on-demand group was nearly twice of that in the secondary prophylaxis group because the number of joint bleeds was much higher in the on-demand group. Therefore, the difference in the changes in the Pettersson scores in these patients was caused only by changes in the number of joint bleeds, depending on the treatment methods. In addition, these results showed that secondary prophylaxis may be effective, even though prophylaxis was started after the late adolescent period.
3. Dosing regimen for prophylaxis
1) Dosing regimen for primary prophylaxis
The intermediate-dose regimen, which included regular injections of 20.25 IU/kg of FVIII CFC every 3 days, was delivered to all patients receiving prophylaxis, including patients receiving primary prophylaxis in the present study due to the restriction of resources in Korea. This intermediate-dose regimen completely prevented joint bleeds in the 2 patients who received primary prophylaxis for 1 year in the present study, which suggests that this dose may completely prevent hemarthropathy in some patients, although the phenotypes and pharmacokinetics in these patients were not evaluated. The standard regimen of prophylaxis for patients with hemophilia A is regular injections of 20-25 IU/kg of FVIII CFC every 48 hours in order to maintain the trough factor activity higher than 1%4,5). This high-dose regimen has been known to completely prevent hemarthropathy when it is started at an early age. However, the cost burden, which is mainly due to the increased FVIII CFC consumption, is the main obstacle to this treatment4,5,11).
The International Society of Thrombosis and Haemostasis defined the severity of hemophilia based on factor activity21). However, there are differences in the coagulation abilities in patients with severe hemophilia, and 10-15% of patients with severe hemophilia A rarely bleed spontaneously even though their factor activity is lower than 1%14,15,22). The preventable levels of factor activity are also different, depending on the stage of arthropathies and activities of the patients23). Therefore, strictly maintaining trough factor activity higher than 1% by using the standard regimen is not an absolute requisite for successful prophylaxis23,24). Furthermore, the pharmacokinetics in individual patients differs, depending on age, body weight, and blood types25-29). Therefore, optimal prophylaxis can be accomplished when the dosing regimens are based on the pharmacokinetics in individual patients, and clinical response such as the observed number of breakthrough bleeds during the prophylaxis is a more important index for successful prophylaxis than just maintaining trough factor activity higher than 1%24,26-28).
2) Dosing regimen for secondary prophylaxis
The dosing regimens for secondary prophylaxis should be different, especially in countries with limited resources. In the present study, the intermediate-dose regimen was delivered to all patients in the secondary prophylaxis group. Although complete prevention of all bleeds was not accomplished in these patients, the frequency of total and joint bleeds decreased markedly in patients in the secondary prophylaxis group; consequently, the increase in the Pettersson score was less in the patients in the secondary prophylaxis group; further, the number of work/school days lost was markedly decreased.
The aim of secondary prophylaxis was to delay the progression of hemarthropathy as much as possible because some stage of hemarthropathies was inevitable in these patients. However, the final acceptable level of arthropathy is still not clear and may differ in different countries. The ultimate goal of all hemophilia treatments is to enhance the QoL for patients. Therefore, it is necessary to know the degree of contribution of arthropathies to QoL in order to determine the final acceptable levels of hemarthropathies in these patients. Thus far, to our knowledge, only 1 study has been conducted on this issue. According to the study30), hemarthropathy was only related to the physical function parameter of the QoL, and the QoL of patients with Pettersson scores between 5.0 and 27.0 was not significantly different from that of patients with Pettersson scores between 0.0 and 4.0. This means that certain levels of hemarthropathy may be acceptable and complete prevention of bleeds with a high-dose regimen may not be necessary in countries with limited resources. The minimum required dosing for prophylaxis was known to be approximately 12 IU/kg of FVIII CFC thrice weekly31), and intermediate regimens comprise regular infusion of 15-25 IU/kg of FVIII CFC twice and thrice weekly. According to the results of comparisons of the effect of prophylaxis between high- and intermediate-dose prophylaxis, the frequency of bleeds was lower, and the percentage of patients without hemarthropathies was higher with the high-dose regimen11). However, changes in the Pettersson scores during the prophylaxis period were not significantly different between patients who received highand intermediate-dose prophylaxis. Furthermore, the QoL of patients was not different, although the consumption of factor concentrates was 2.19 times higher with the high-dose regimen11). Therefore, an intermediate-dosing regimen could be acceptable, especially in countries with limited resources that cannot afford high-dose prophylaxis.
In conclusion, to our knowledge, this is the first prophylaxis study on Korean patients with severe hemophilia A; the beneficial effects of intermediate-dose prophylaxis were observed in these patients, in spite of the limitations of this study, such as number of patients in each group and the relatively short period of observations. Intermediate-dose prophylaxis may prevent hemarthropathy by complete prevention of all joint bleeds when it is started at an early age. Secondary prophylaxis with the same intermediate-dose could delay the progression of hemarthropathy in Korean patients by reducing the frequency of joint bleeds, even in patients older than 17 years. However, modifying the dosing regimens depending on pharmacokinetics and clinical responses in individual patients is warranted.
Notes
No potential conflict of interest relevant to this article was reported.