Current issues of pediatric inflammatory bowel disease in Korea
Article information
Abstract
Inflammatory bowel disease (IBD) is a chronic relapsing disorder of unknown etiology, which is believed to be multifactorial. Recently, the incidence of pediatric IBD has steeply increased in Korea since 2000. Poorly controlled disease activity can result in complications such as intestinal fistulae, abscess, and stricture, as well as growth retardation and delayed puberty in children. Because of a lack of confirmative tests, various diagnostic modalities must be used to diagnose IBD. Onset age, location, behavior, and activity are important in selecting treatments. Monogenic IBD must be excluded among infantile and refractory very-early-onset IBD. Early aggressive therapy using biologics has recently been proposed for peripubertal children to prevent growth failure and malnutrition.
Introduction
Pediatric inflammatory bowel disease (IBD) was previously rare in Korea1); therefore, most general pediatricians in Korea may have encountered a few cases of pediatric IBD during their training program. However, the incidence of IBD has risen in adults since 19902) and in children in the 2000s3,4), and its incidence continues to increase without having reached a peak. The incidence of IBD has been rapidly increasing in the last 5 years. General pediatricians are expected to encounter children with IBD more frequently in the near future. To improve the understanding of pediatric IBD among Korean pediatricians, we have written this review focusing on the issues that may pertain to clinical practice in Korea.
What is IBD?
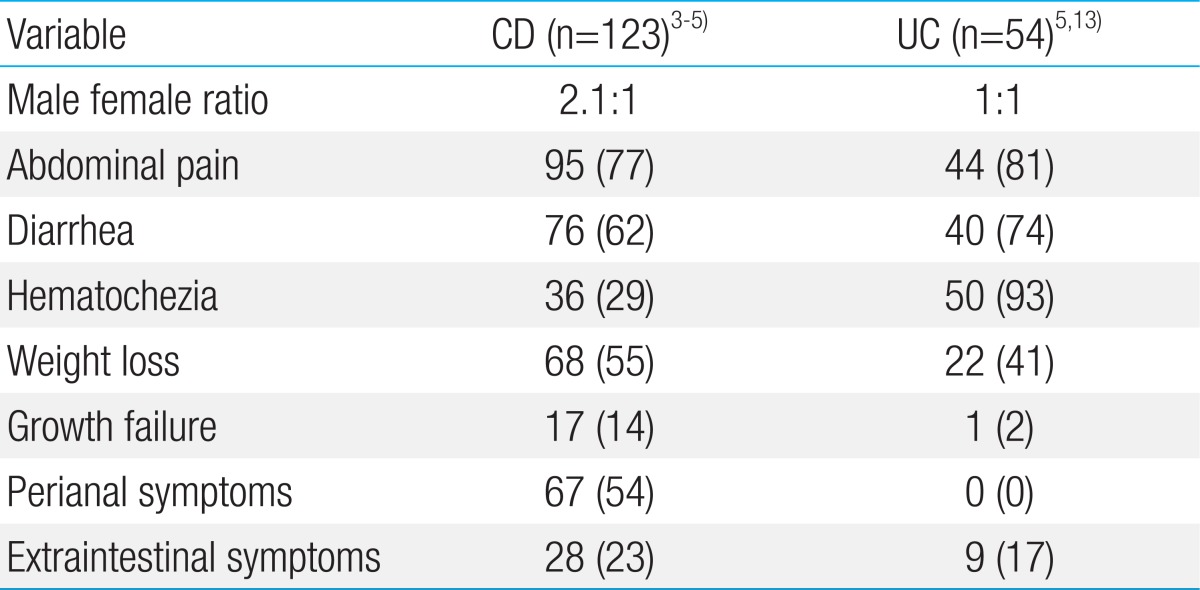
IBD is a chronic relapsing disorder of unknown etiology that encompasses the two distinct disorders of Crohn disease (CD) and ulcerative colitis (UC). In CD, inflammation can occur anywhere in the gastrointestinal tract. CD, although rarely fatal, is refractory and causes abdominal pain, diarrhea, anorexia, and weight loss (Table 1)3,4,5). The clinical presentations of Korean children with CD were similar that observed in Western countries6,7,8). In our study, weight loss, growth retardation, lower bone mineral density, and nutritional deficiencies were also observed9) (Table 2). Importantly, growth failure is one of the most important aspects in pediatric CD10). Unlike in the adult population, disease onset in children is uniquely associated with growth retardation and delayed puberty. Poor control of disease activity can result in serious complications such as intestinal fistulae, abscess, and stricture, requiring surgery in both children and adults. The incidence of complications of IBD reported in adult Koreans is lower than that in the West; however, the rate of resection 10 years after the initial diagnosis has recently risen to 32.8%11). In our report, 30.0% of children with IBD required surgeries 10 years after the initial diagnosis12). There is currently no medical cure, and the disease is considered a rare, intractable condition that persists throughout a patient's lifetime. Most children with UC in Korea present with hematochezia and diarrhea at the time of diagnosis5,13), in line with the observations in Western studies6,7,8). Based on the nature of chronic relapsing IBD, early-onset IBD can greatly lower the quality of life, including an inability to work and social maladjustment, in children with IBD. Therefore, better awareness and effective measures for treating pediatric IBD are needed at a societal level.
How does IBD develop?
Understanding of the pathogenesis of IBD is essential for identifying several key points at which intestinal inflammation can be modulated and expanding our treatment options for IBD. The pathogenesis of the disease involves the complex interactions between genetic factors14), environmental factors15,16) such as commensal intestinal bacteria, and immunological factors17). The discordance of IBD among monozygotic twins18) and the increased incidence of IBD among immigrants to high-prevalence countries indicate an important role of environmental factors in the development of IBD. These factors may cause disruptions in the intestinal microbiota (dysbiosis) and modify the relationships between the human intestinal microbiota and mucosal immune system. The enormous success of tumor necrotic factor-α blockade in IBD treatment, which opened a new era of investigation for the pathogenesis of IBD, suggests that IBD is an autoinflammatory disease of the intestine.
In addition, more than 100 genetic loci, of which variants may alter normal mucosal immunity in the gastrointestinal tract, were uncovered by genome-wide association studies (GWASs) in Western populations19,20,21). Recently, a Korean GWAS also identified an extensive overlap of genetic risks with those of Western populations22). GWASs of pediatric IBD are rare, and only two pediatric GWASs have been conducted, with the studies noting several new loci23,24). However, those loci were replicated in large-scale GWAS meta-analyses in adult populations21). Interestingly, despite an extensive overlap of genetic risk with Western populations, nucleotide-binding oligomerization domain (NOD2/CARD15) and autophagy-related 16-like 1 (ATG16L1) have not been associated with IBD in the Korean population, indicating ethnic heterogeneity22). In addition, we uncovered three new susceptibility loci (ATG16L2, DUSP5, and TBC1D1) in a GWAS of Korean adults and children with IBD. Our other study on pediatric genetics did not find any association with NOD2 mutation25). Variations of TNFSF15, which was demonstrated to have a weak association with IBD in Western populations, was found to greatly contribute to IBD susceptibility in both adults and children in Korea26). This further supports the existence of ethnic differences in the genetics of IBD between populations.
Is the incidence of IBD increasing in Korean adults and children?
The global incidence of pediatric CD is not known27). The annual incidence of pediatric CD in Europe and North America is 0.2-8.5 cases per 100,000 people, and that of UC is 0.5-4.3 cases per 100,000 people. Approximately 25%-30% of all cases of CD and 20% of all cases of UC occur in subjects less than 18 years old28). Approximately two decades ago, IBD was considered a rare disease among Korean adults. However, the incidence of IBD started to rise rapidly after the 1990s. A study examining the frequency of IBD in Korean adults illustrated that the prevalence of IBD was 0.05 cases per 100,000 adults in the Gangdong and Songpa districts of Seoul before 19902), whereas it increased to 1.34 cases per 100,000 adults after 2000. This steep upward trend in the Korean population is reported to be ongoing. This population-based study did not include a national sample; however, it clearly revealed that the incidence of IBD is on the rise in Korean adults. The onset of IBD is reported to be extremely rare in Korean children1); however, recent single-center studies confirmed that the number of pediatric patients newly diagnosed with CD has increased since 20003,4). A population-based study in children is essential to accurately determine whether the incidence of IBD is rising continuously in Korea.
Does the clinical manifestation of IBD in Korean patients differ from that in the West?
In our study, most patients with CD (74%) presented with both small bowel and colonic involvement, whereas isolated ileal (13%) and colonic (13%) disease was rare3), as also reported in the Western study of Sawczenko and Sandhu7), which found that most patients (84%) had both ileal and colonic involvement and that the dual presentation was more common than observed in a previous report29). These authors considered that isolated colonic disease was more common in the younger children of our study, as noted in a Western report30).
Regarding the location of disease in Korean adults with CD, 66.7%-75.7% of patients had CD in both the small intestine and colon, 10.8%-25.9% exhibited disease in the small intestine, and 7.4%-13.5% displayed disease in the colon, in line with the findings in the pediatric population2). The relatively higher rate of CD in the small intestine and colon as well as the markedly low rate of colonic CD indicates that ethnical differences exist in terms of disease location in adult patients with CD. Moreover, the rate of anal fistula was higher in Korean adults than in Western populations. A study on Korean adults reported that anal fistula was the initial symptom in 15.8% of patients, and the accumulative incidence of anal fistula over 10 years was 49.7%, which is markedly higher than the rates of 13%-38% reported in the West11,31). Our study3) also found a higher incidence (50%) of perianal fistula than noted in Western children, including rates of 8% in the work of Kugathasan et al.6) and 15% in the study by Palder et al.32).
Infantile and very-early-onset IBD
According to the Paris classification for pediatric IBD, IBD in subjects less than 10 years old is classified as very-early-onset (VEO) IBD33). VEO IBD often involves isolated colitis34). The prevalence of anti-Saccharomyces cerevisiae antibody (ASCA) and anti-CBir1 increases and decreases, respectively, until the age of 10 years33). As VEO IBD has more severe phenotypes and a greater family history, the disease has been postulated to have highly penetrant mutations of Mendelian-like disease. A case of infantile IBD with an IL10R mutation was the first case of a Mendelian form of monogenic IBD35). A recent Korean study also illustrated that as many as 50% of subjects with infantile IBD carried IL10RA mutations36).
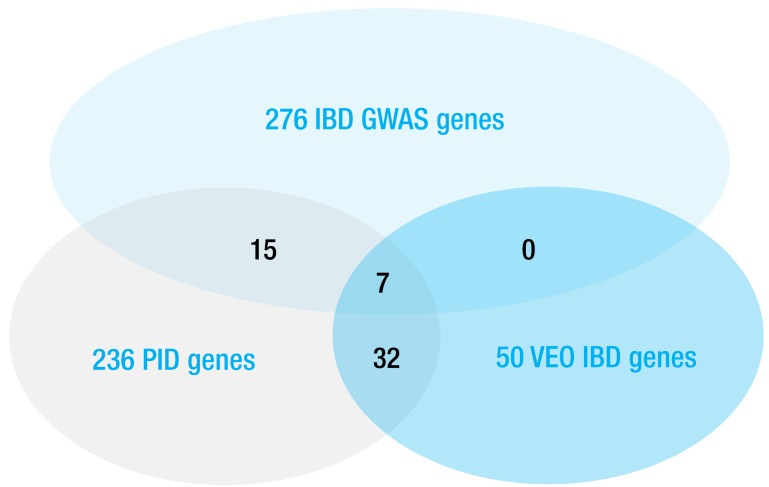
Recently, two reviewers identified approximately 50 genes associated with monogenic IBD37,38). Interestingly, three-fourths of these genes are known to be associated with primary immunodeficiency39), for which treatments have not been established with the exceptions of hematopoietic stem cell transplantation and repeated immunoglobulin infusion (Fig. 1). In addition, the diagnosis of these heterogeneous diseases is also challenging because of the limits of conventional evaluations for immunodeficiency in clinical practice. Therefore, whole-exome sequencing (WES) is recommended to diagnose monogenic IBD to avoid a "diagnostic odyssey"37,38). A case of infantile IBD featuring an XIAP mutation was the first example of the application of WES for diagnosing monogenic IBD40). However, interpreting variants of unknown significance in WES remains challenging.
Diagnosis and classification of IBD
No single unique test or surrogate markers that can diagnose IBD alone exist; therefore, the diagnosis of IBD can be made according to the portfolio for suspected patients through history taking, physical examination, and laboratory, radiologic, endoscopic, and histologic criteria41,42). Detailed diagnostic guidelines were devised for adult Korean patients with IBD43,44). Those for pediatric Korean patients have not been created, although they should become available in the near future. First, history taking, physical examination, and laboratory tests for anemia and inflammatory markers should be performed. Then, infectious enteritis or colitis should be excluded. In addition, Korea is an endemic area of tuberculosis; therefore, tuberculosis should be excluded cautiously in children with IBD.
All patients should undergo a full colonoscopy with ileal intubation and mucosal biopsy for histology and culture. We perform esophagogastroduodenoscopy at the time of diagnosis, in addition to radiographic methods such as small bowel series, barium enema, abdominal computed tomography (CT), ultrasonography, and magnetic resonance enterography (MRE) to determine the lesion location and behavior. In the pediatric population, MRE is preferred to CT, as it involves no radiation exposure and it is useful for dynamic studies. MRE is particularly useful in assessing the small intestine; however, we perform capsule endoscopy when MRE alone is not sufficient to confirm lesions confined to the small intestinal mucosa.
The initial evaluation of disease behavior and locations as well as disease activity is important, as it can affect the treatment regimen or selection of medical therapies. We classify the disease status according to the Paris Classification33). Disease location and behavior such as inflammatory, fistulizing, and stricturing are determined at diagnosis and during follow-up. The presence of any macroscopic abnormality, such as mucosal ulceration, fistula, stricture, or abscess, was considered to indicate regional involvement. The perianal lesions of patients with IBD also include skin tags, fissures, fistulas, and abscesses. In addition, disease activity should be assessed at the time of diagnosis according to the Pediatric Crohn's Disease Activity Index (PCDAI) for CD, which is based on symptoms (30%), physical examination (30%), laboratory parameters (20%), and growth data (20%)45). PCDAI scores range from 0 to 100, and they are categorized as follows: no disease activity (<10), mild disease activity (11-30), and moderate to severe disease activity (≥30)46). The Pediatric Ulcerative Disease Activity Index (PUCAI) for UC should be also assessed47). PUCAI scores range from 0 to 85, and they are categorized as follows: no disease activity (<10), mild disease activity (11-34), moderate disease activity (35-64), and severe disease activity (≥65). In addition to these diagnostic tools, bone densitometry and an evaluation of nutritional status are also needed.
What is the goal in the treatment of IBD?
In the past, because of the lack of treatment options, the goal of treating IBD was symptom relief to improve quality of life. In children, an additional goal was to prevent growth retardation48). However, the recent introduction and aggressive use of biologics has improved patient responses. At present, the primary goal of treatment goes beyond clinical remission to mucosal healing on endoscopy49). The endoscopic severity is recognized as the most accurate predictor of forthcoming surgery. It is well known that there is a discrepancy and poor correlation between clinical severity and endoscopic severity50). Mucosal healing may not be predicted by CDAI or PCDAI. According to the current understanding of the disease, mucosal damage is accumulated regardless of the clinical disease activity to inflict permanent gastrointestinal damage with a stricturing or penetrating disease51). Studies in children disclosed similar results. During a mean follow-up period of 36 months, 20% of patients progressed to a stricturing and penetrating disease52).
Step-up therapy has been the mainstay of the treatment algorithm, in which the primary treatment was determined according to the disease activity and behavior to be gradually strengthened when there was no response. Recently, top-down therapy has been considered more effective in patients with at least disease moderate activity53,54). However, more scientific evidence is needed to support the use of universal top-down therapy in children. To compensate for the shortcomings of both strategies, targeted early aggressive therapy has recently been proposed. This is a more individualized approach to treating IBD. Siegel et al.54) reported that the early use of biologics in high-risk pediatric patients could reduce complications by more than 75%. However, Dubinsky et al.55) argued that early aggressive therapy should be conducted in certain patient populations after assessing clinical, immunological, and genetic risk factors. Pediatric patients with poor early responses who enter puberty can experience potential growth retardation before displaying a response to therapy. Top-down therapy may be more beneficial in those patients to minimize the peripubertal accumulation of intestinal damage and avoid underdevelopment from inadequate therapy.
Currently, clinical, serological, and genetic risk factors are being extensively studied to facilitate patient selection. Previously known clinical risk factors of surgical resection of the small intestine and complications include extensive involvement of the small intestine, perianal fistula, young age at diagnosis, and the use of steroids56). The presence of antibodies to three bacterial antigens (ASCA, anti-OmpC, and anti-CBir1) has also been reported to be associated with prognosis52). Studies in Western populations reported an association between variations in NOD2/CARD15 with prognosis20); however, this association was not supported in Korean studies25,57). Henckaerts et al.58) reported over 30 genes associated with susceptibility to IBD. We also found in our study that TNFSF15 was associated with clinical manifestations26). Detailed reviews of the management of IBD were described previously48,59). Doctors need to be aware of the rapid changes in the understanding of the pathogenesis and the rapid development and introduction of newer drugs. For example, for anti- tumor necrosis factor-α, as the U.S. Food and Drug Administration (FDA) continues to monitor the safety of this drugs, doctors should pay attention to the results that they are providing (www.fda.gov/cder/drug/early_comm/TNF_blockers.htm).
Growth and nutrition in pediatric CD
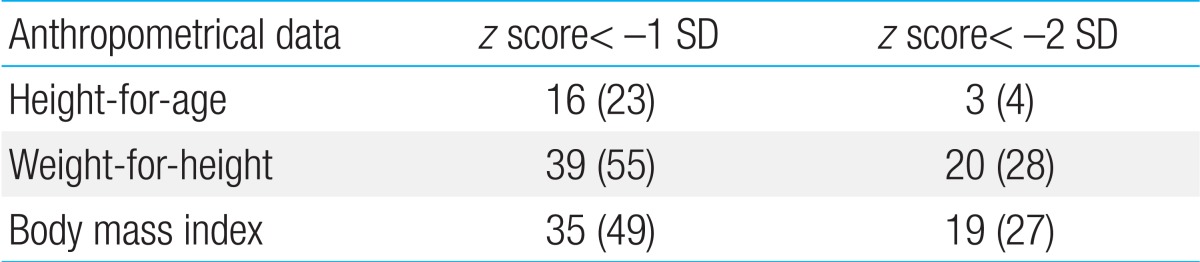
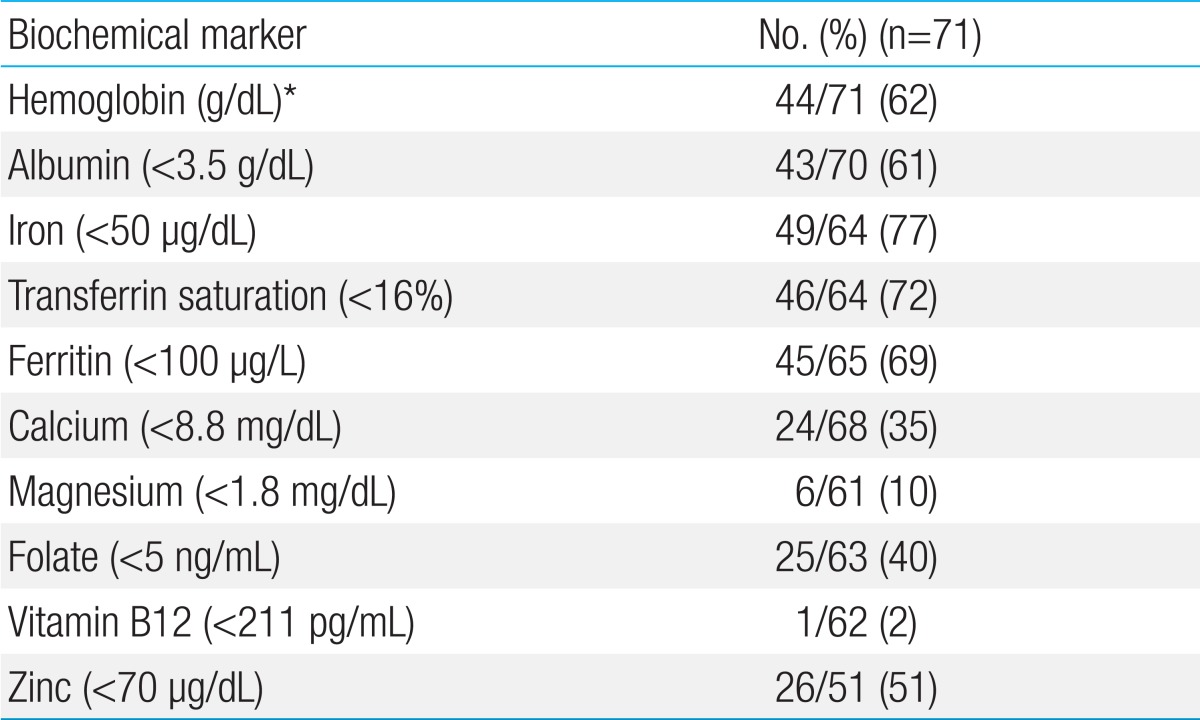
Growth failure is one of the most important aspects of pediatric CD10). The decrease in height velocity usually occurs before the onset of gastrointestinal symptoms. One-fourth of patients with CD and growth retardation may not achieve their full adult height potential. Therefore, interventions should be initiated before the completion of puberty. First, the disease should be controlled. Second, we must optimize nutrition and minimize corticosteroid use60). Interventions are effective if performed in prepubertal patients or in the early stages of puberty. The prevalence of growth retardation at the time of diagnosis in Korean children is approximately 5%, which is less than that reported in a Western report9). Severe weight loss and low body mass index were observed in approximately one-third of patients. One-fifth of children already have osteoporosis at the time of diagnosis. In addition, altered nutrition markers for macronutrients as well as micronutrients were highly prevalent at time of diagnosis of CD in Korean children (Table 3).
Conclusions
Understanding the unique features of pediatric IBD is important for its diagnosis and management. First, some clues to the pathogenesis of the disease can be uncovered when we understand the reasons of the current change in the incidence or prevalence between different regions and ethnic backgrounds. Second, we must consider different management strategy when pediatric patients have different characteristics compared to adults.
Poorly controlled disease activity can result in inevitable surgery and growth retardation in children with IBD. Various diagnostic methods in the diagnosis and treatment of IBD have been used to evaluate the locations, behavior, and activity of the disease. Monogenic IBD must be excluded among patients with infantile and refractory VEO IBD. Recently, early aggressive therapy using biologics has recently been proposed for children with IBD who are at great risk of growth failure and malnutrition. A population-based study is needed to accurately estimate the incidence of pediatric IBD in Korea.
Notes
No potential conflict of interest relevant to this article was reported.