Long-term effects of gonadotropin-releasing hormone analogs in girls with central precocious puberty
Article information
Abstract
Gonadotropin-releasing hormone analogs (GnRHa) are widely used to treat central precocious puberty (CPP). The efficacy and safety of GnRHa treatment are known, but concerns regarding long-term complications are increasing. Follow-up observation results after GnRHa treatment cessation in female CPP patients up to adulthood showed that treatment (especially <6 years) was beneficial for final adult height relative to that of pretreated or untreated patients. Puberty was recovered within 1 year after GnRHa treatment discontinuation, and there were no abnormalities in reproductive function. CPP patients had a relatively high body mass index (BMI) at the time of CPP diagnosis, but BMI standard deviation score maintenance during GnRHa treatment seemed to prevent the aggravation of obesity in many cases. Bone mineral density decreases during GnRHa treatment but recovers to normal afterwards, and peak bone mass formation through bone mineral accretion during puberty is not affected. Recent studies reported a high prevalence of polycystic ovarian syndrome in CPP patients after GnRHa treatment, but it remains unclear whether the cause is the reproductive mechanism of CPP or GnRHa treatment itself. Studies of the psychosocial effects on CPP patients after GnRHa treatment are very limited. Some studies have reported decreases in psychosocial problems after GnRHa treatment. Overall, GnRHa seems effective and safe for CPP patients, based on long-term follow-up studies. There have been only a few long-term studies on GnRHa treatment in CPP patients in Korea; therefore, additional long-term follow-up investigations are needed to establish the efficacy and safety of GnRHa in the Korean population.
Introduction
If not diagnosed and treated at an early stage, central precocious puberty (CPP) can compromise final adult height, cause incongruity between psychological and physical development, and may also trigger psychological problems arising from early menarche. Gonadotropin-releasing hormone analogs (GnRHa) have been widely used for more than 30 years to solve these problems in patients with CPP1). Although the safety and efficacy of GnRHa are known, the treatment dosage and duration and point of cessation vary among different countries and ethnic groups, and the reported results are inconsistent because of many variables, including genetic height, pubertal traits, and treatment response. An analysis of Korean CPP patients from 2006-2010 conducted by the Health Insurance Review and Assessment Service showed an average 44.4% annual increase in patients diagnosed with CPP and a 4.5-fold increase in patients treated with drug therapy during the last 5 years2). Likewise, concerns about the long-term side effects of GnRHa treatment are increasing along with the rapid increase in patients diagnosed with CPP and receiving subsequent treatment. However, the long-term effects of GnRHa treatment remain controversial. This paper reviewed long-term data up to adulthood from both international and Korean studies regarding final adult height, reproductive function, body composition, polycystic ovary syndrome (PCOS), and bone and psychological outcomes in girls with CPP after GnRHa treatment. As long-term studies of male CPP patients are scarce, this paper only addressed female CPP patients.
Final height
The effect of GnRHa treatment on final height (FH) gain is well known, but the degree of height gain varies among studies. This variance may result from differences in the adult height prediction methods, which include comparing adult heights between GnRHa treated and untreated groups or comparing the target height with predicted adult height (PAH) before and after treatment according to the Bayley-Pinneau method. In addition, differences in height gain may be induced by the delayed onset of treatment after diagnosis, duration of treatment, and age at the start of treatment.
Regarding the timing of CPP treatment discontinuation, Carel et al.3) reported that the final adult height increased when GnRHa treatment was discontinued at 11 years of age but decreased when GnRHa treatment was continued beyond that age. According to bone age standards, 12-12.5 years is the suggested appropriate age at which to discontinue treatment in order to reach the maximum adult height4). However, the psychological states of the patients and their families, as well as the proposed age for recovery of the optimal adult height and bone age, must be considered before deciding whether or not to discontinue treatment.
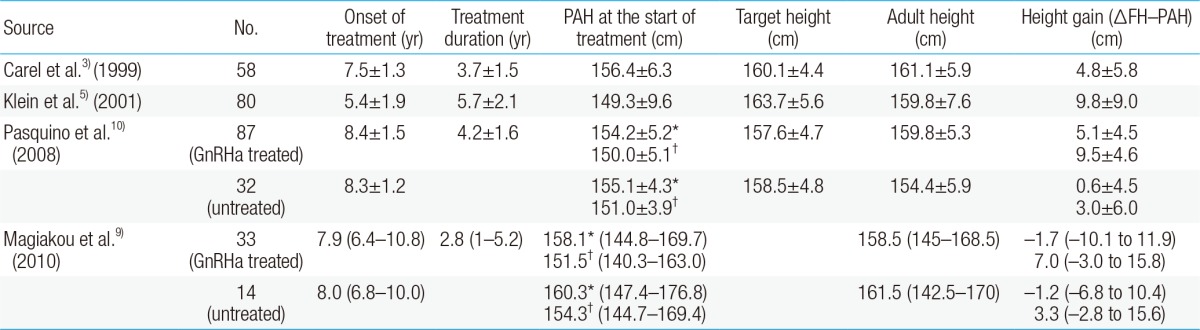
Long-term investigations have reported results from objective evaluations of the effects of GnRHa treatment on final adult height. Klein et al.5) reported the effect of GnRHa treatment on final adult height based on a sample of 98 males and females patients with growth cessation after GnRHa treatment. In that study, the final adult height increased significantly relative to the PAH before treatment after GnRHa treatment in 80 female subjects, although not sufficiently to reach the target height; 18 male subjects also had similar outcomes. However, as these patients achieved their target heights when they started treatment within 2 years of diagnosis, the authors concluded that rapid treatment after early diagnosis and, by extension, long-term treatment would be beneficial for achieving a maximum final adult height.
Some studies show that the effect on final adult height differs depending on the onset of CPP treatment. GnRHa treatment is known to maximally affect final adult height when initiated before 6 years of age6) but the effect is decreased after age 6 years7). However, Carel et al.3) compared the effects of starting treatment before or after age 6 years on the FHs of CPP patients. The results indicated that the under 6 group and older group had height gains of 5.3±7.2 cm and 4.5±5.3 cm, respectively, compared to the PAH, and that the FH gain therefore had no relationship with the onset of puberty or the age of treatment onset. On the other hand, in a study that stratified patients by age and compared their height gains after treatment, the age 6-8 groups and age 8-10 groups had final adult heights shorter than the PAH at the time of treatment discontinuation, but the age below 6 group had similar final adult heights relative to the PAH at the time of treatment discontinuation8). Also, a recent study evaluated a group of patients aged 16-32 years who had previously received GnRHa treatment and found no difference in the FH between subjects who had begun receiving treatment at approximately 8 years and other subjects who had not received any treatment9). It appears that patients experience the benefit of height gain from GnRHa treatment only if treatment is initiated before age 6. In other words, patients can have a longer period of growth following long-term treatment, allowing the acquisition of genetic height.
However, in a study of CPP patients who had received treatment 10 years earlier at an average age of 8.4 years, the adult height increased significantly compared to the PAH before treatment, and the patients reached or exceeded their target heights. Also, when the treated group was compared with the untreated group, the adult height of the untreated group was an average of 5.4 cm shorter than that of the treated group and 4.1 cm shorter than the target height10). Currently, children enter puberty at younger ages and therefore some argue that the diagnostic standard of CPP in females should accordingly be lower than age 8. However, GnRHa treatment is still needed to increase the FH in CPP patients under the age of 8 years.
Currently, there are few long-term studies on the final adult heights of CPP patients in Korea. Kwon et al.11) reported that after tracking CPP patients who had received an average of 2.5 years of GnRHa treatment for approximately 3.4 years, the PAH increased by approximately 5 cm in 2 years and approximately 6-7 cm in 3 years after treatment11). Another study showed that the near adult height increased by approximately 5.1 cm, compared with the PAH in CPP patients after GnRHa treatment12). A long-term study involving Korean CPP patients is needed to examine the effect of treatment on final adult height gains.
In CPP, posttreatment growth evaluation is often performed using the difference between the FH and PAH according to the Bayley-Pinneau method. One study reported that when calculating the PAH, using the average table from the Bayley-Pinneau method reduced potential measurement errors from PAH overestimation when compared with using the accelerated table from the Bayley-Pinneau method13). However, in a recent study, there was no significant difference in the change in FH (ΔFH)-PAH of the untreated group when the values were calculated using either the accelerated or the average table from the Bayley-Pinneau method; in the GnRHa-treated group, the average table ΔFH-PAH (ΔFH-PAHav) was significantly higher than the accelerated table ΔFH-PAH (ΔFH-PAHacc)9) (Table 1). Therefore, it is more appropriate to use the accelerated table when evaluating the FH in CPP patients after treatment. However, instead of evaluating the PAH at a certain point, periodic height, growth rate, pubertal progress, and bone age evaluations should be conducted so that optimal height gain can be achieved.
Height outcomes in girls with central precocious puberty who received gonadotropin-releasing hormone agonist therapy
In a study of factors affecting FH gain, early diagnosis and treatment were found to be essential because bone age progression and treatment delay are known to adversely affect height gain. Furthermore, the degree of bone age progression, decrease in growth velocity, and low PAH at treatment cessation have been acknowledged as factors that negatively affect FH acquisition14).
In the case of rapidly progressive precocious puberty, early GnRHa treatment is necessary; however, as the adult height can reach the target height without GnRHa treatment in slowly progressive CPP, side effects resulting from unnecessary GnRHa treatment should be considered15,16).
Time of menarche after treatment discontinuation and reproductive function
Long-term studies on the recovery of reproductive function in CPP patients of more than 6-20 years are being reported. In CPP patients, pubertal reactions were recovered within 1 year and mostly within 6 months after treatment discontinuation. An average interval of 0.9-1.5 years was required for the onset of menarche after treatment cessation, and menarche started at approximately 12.6-13.6 years old in chronologic age. In a Korean study, Baek et al.12) reported that menarche began at approximately 14 months post-GnRHa treatment, which was 11.9 years in chronologic age and 12.8 years in bone age; these ages are similar to the average age at menarche among normal girls in Korea (12.6 years). When patients were stratified according to their age at CPP diagnosis (age under 6 groups, age 6-8 groups, and age 8-9 groups), the repuberty period and onset of menarche after treatment were similar regardless of the age at diagnosis8,10,17). These results demonstrate that older CPP patients may have a shorter final adult height than younger patients because of the shorter period of time remaining for height gain after GnRHa treatment.
Among CPP patients, the average age at menarche in the GnRHa-treated group was 12 years, a significant delay when compared with the age of 9.6 years in the untreated group9). Therefore, untreated CPP can cause psychological problems due to early menarche. These studies demonstrated that the average age at menarche after GnRHa treatment was similar to the age at menarche in normal, healthy girls.
Menstrual cycles and pregnancy outcomes were analyzed to evaluate reproductive function. Pasquino et al.10) reported that among 87 GnRHa-treated patients, 82 had regular menses, 5 had oligomenorrhea caused by excessive exercise but recovered after controlling their exercise, and 6 achieved pregnancy and normal childbirth. Neely et al.17) reported that among 20 CPP patients who had reached adulthood, 80% had normal posttreatment menstrual cycles and 7 patients achieved a total of 12 pregnancies; these patients experienced normal childbirth and a number of miscarriages similar to that of the general population. Heger et al.18) investigated 34 patients (average age, 23.6 years) who were followed up for an average of 12.5 years after treatment and wished to become pregnant. Twelve of these patients became pregnant. The patients had regular menses cycles and did not have any breast or uterine disorders. Therefore, these results demonstrate that the reproductive function of women treated for CPP does not different from that of normal, healthy women.
Obesity and metabolic syndrome
Genetic factors, nutritional status, obesity, and other environmental factors contribute to the onset of puberty. In particular, a certain level of lipid accumulation in the body is required for the onset of puberty, and adipocyte-secreted leptin stimulates the release of gonadotropin-releasing hormone from the pituitary gland, thereby increasing sex hormone secretion. Breast development and menarche are known to be accelerated in women with high BMI values19). Furthermore, the risks of metabolic syndrome such as adult obesity, cardiovascular disease, and diabetes were reported to be higher in patients with early menarche20). In other words, childhood obesity may induce early puberty and early menarche and may lead to metabolic problems during adulthood. Likewise, there is increasing interest in changes in BMI or metabolic risk factors in CPP patients before and after GnRHa treatment.
To date, the reported results of research on changes in the BMI values of CPP patients before and after treatment are inconsistent. Paterson et al.21) reported that the mean BMI SD scores (SDS) of CPP patients increased from 0.93 to 1.2, the frequency of overweight patients increased from 41% to 59% of all patients, and the frequency of obese patients increased from 28% to 39%. Arrigo et al.22) reported that 23.8% of their CPP patients were obese prior to GnRHa treatment but experienced BMI decreases after at least 2 years of treatment. Another study showed that GnRHa treatment did not aggravate obesity, as CPP patients maintained their previous BMI SDS during treatment regardless of the overall increase in BMI after GnRHa treatment10,23). Many CPP patients were obese prior to GnRHa treatment but experienced no changes in BMI SDS following treatment, and the BMI SDS before treatment correlated strongly with the BMI SDS after treatment discontinuation24). From a comparison of a GnRHa-treated group and a nontreated group among adult CPP patients, Magiakou et al.9) reported no difference in the BMI SDS between the 2 groups. Furthermore, as no difference in body fat mass via dual-energy x-ray absorptiometry was found between the 2 groups, it appears likely that GnRHa treatment is not associated with an increase in fat mass.
Research on the metabolic risk factors following GnRHa treatment in CPP patients is limited. In a previous study, reduced insulin sensitivity and lipid disorder incidence were observed in CPP patients at diagnosis, and metabolic disorders were aggravated in these patients during a 1-year GnRHa treatment period25). Another report indicated that increased insulin resistance after GnRHa treatment could lead to metabolic problems in CPP patients26). Yet another study showed that despite a lack of change in the BMI SDS during GnRHa treatment period, CPP patients had a 2-fold increase in total fat mass compared with a normal population after reaching their FH after treatment23). Currently, there is a relatively low amount of research concerning changes in body composition and metabolic profiles in CPP patients following GnRHa treatment, but adequate education concerning lifestyle and diet after treatment is needed in addition to medical treatment during the GnRHa treatment period.
Bone mineral density and bone markers
During the pubertal growth spurt, children experience improved bone mineral accretion, and almost half of the adult peak bone mass (PBM) is accumulated during this period. Genetic factors, hormonal status (growth hormone/insulin-like growth factor-1, sex steroids), nutrition, and physical activities are factors that influence PBM27). Estrogen in particular is well known as an important factor in bone mineralization and development. Reduced bone mass consequent to decreased estrogen levels along with a high occurrence of osteoporosis in menopausal women was reported in adults who had received GnRHa treatment28). Therefore, there is growing concern that missing the critical PBM stage might have adverse effects on adult bone, as the estrogen concentration is decreased during GnRHa treatment. In an earlier study, bone mineral density (BMD) decreased in CPP patients during GnRHa treatment29). In a study comparing BMD between a GnRHa-treated group and GnRHa plus calcium supplementation-treated group, Antoniazzi et al.30) reported that although the BMD decreased during GnRHa treatment, this was reversible and preventable with calcium supplementation. However, another study reported a normal BMD in CPP patients who had reached their FH after GnRHa treatment. Regarding bone turnover markers in CPP patients, the expression of carboxy terminal telopeptide of type 1 collagen (ICTP), a bone resorption marker, and procollagen type 1 C-terminal propeptide (PICP), a bone formation marker, increased prior to GnRHa treatment but decreased during a 6-month treatment period and stabilized after treatment. Bone age-adjusted bone turnover markers were also normalized 2 years after treatment cessation. Furthermore, a report indicated that no changes in age- and bone age-adjusted BMD SDS was observed during GnRHa treatment31). A study conducted in Korea observed no changes in bone maturation among CPP patients who had received 3 years of GnRHa treatment32). In another study, bone mineral accumulation was suppressed during GnRHa treatment; however, the bone mineral content was recovered and PBM was sufficiently achieved when the adult height was reached after treatment discontinuation10).
The varying BMD findings after GnRHa treatment might be explained by differences in age, treatment duration, treatment dosage, BMD area of measurement, and BMD evaluation method. However, to summarize the long-term BMD studies in CPP patients, although BMD levels decreased during GnRHa treatment, the bone mass was sufficiently preserved after treatment and treatment seemed to have no adverse effects on bone. As in normal girls and adolescents, exercise and adequate nutritional intake would be helpful for bone mass formation in CPP patients.
Polycystic ovary syndrome
PCOS is observed in 5%-10% of women of reproductive age and is characterized by anovulation, hyperandrogenism, and polycystic ovaries33,34). Severe insulin-resistant obesity, premature adrenarche, and sexual precocity in childhood are some of the known risk factors of PCOS. The cause of PCOS is not certain, but it may be induced by neuroendocrine system abnormalities that could cause abnormal luteinizing hormone (LH) pulsation and secretion, gonadal steroidogenesis disorders, or hyperinsulinemia35).
Among the many causes of PCOS, hypersecretion of LH relative to follicle-stimulating hormone is especially similar in mechanism to activation of the hypothalamic-pituitary axis in patients with CPP, and some researchers have explained that CPP precedes PCOS through neuroendocrine dysregulation36). Exaggerated adrenarche was observed in 55% of cases at CPP diagnosis, and premature adrenarche was diagnosed via the hypersecretion of 17-hyderoxyprognenolone after adrenocorticotropic hormone stimulation. As 40% of patients diagnosed with CPP also had PCOS during the postmenarcheal period, the risk of PCOS might have increased because of the inherent premature adrenarche in CPP patients. In CPP patients, PCOS is known to usually develop within 0.5-4 years after menarche37).
The prevalence of PCOS among CPP patients varies depending on the characteristics of the patients, durations of treatment and follow-up period, and differences in the PCOS diagnosis standards. Furthermore, a report stated that the prevalence of PCOS among CPP patients was 24%, compared with 2% in an age-matched control group38). On the other hand, Magiakou et al.9) reported that GnRHa treatment had no influence on the occurrence of adult PCOS; rather, the authors concluded that ovarian dysfunction was more likely to occur in GnRHa-untreated patients. Franceschi et al.26) reported the prevalence rate of PCOS in 46 adult women who were previously diagnosed with CPP and received GnRHa treatment. PCOS was diagnosed according to the Rotterdam and Androgen Excess Society definitions in 32% and 30% of the patients, respectively. That study reported that ovarian hyperandrogenism and polycystic ovaries occurred in CPP regardless of GnRHa treatment. However, the evaluation was limited by the lack of a GnRHa-untreated control group. Chiavaroli et al.39) conducted a study on PCOS and compared a GnRHa-treated group with a GnRHa-untreated group among patients with early puberty (8-10 years of age). The authors reported higher prevalence rates of PCOS and hyperandrogenemia in the GnRHa-treated group than in the untreated group and that GnRHa treatment appeared to be an independent risk factor for PCOS occurrence.
Unlike previous studies, recent studies have reported a higher incidence of PCOS among CPP patients, but it is unclear whether this is due to the hyperinsulinemia or premature adrenarche already present at CPP onset or a result of an abnormal hormonal response to GnRHa treatment. A comparison with a control group of CPP patients through a long-term evaluation from diagnosis to posttreatment adulthood is needed to determine the causative factors of PCOS.
Psychosocial problems
Early pubertal timing in adolescents tends to result in severe emotional problems, antisocial behavior, or conflict with parents40). Adolescents who experienced menarche at an early age are known to be at risk for several problems such as excessive drug or alcohol intake, sexual contact at an early age, and increased psychosomatic problems during menstrual periods41,42). However, there are very few studies on the psychosocial problems of CPP patients after GnRHa treatment. A study evaluated the behavior and self-esteem of CPP patients before and during a 2-year GnRHa treatment period and reported that although the patients had been very concerned about physical differences from their peers, they recovered slightly from loneliness and behavior disorders during treatment43). Mul et al.44) reported that there were no emotional or behavioral problems in early puberty patients at the start of or during treatment and there was also no influence on self-perception. In my study, the externalization problem, total behavior, thought, and attention problem scores were higher in CPP patients than in a normal control group, but there were no clinically meaningful behavioral problems45). Body image self-perception had an influence on emotional problems, regardless of a good response to treatment, in CPP patients with pubertal suppression after GnRHa treatment (not submitted). The patient's self-perceived body image, rather than physical improvement after GnRHa treatment, may play a more significant psychological role, and therefore psychological support should be provided during GnRHa treatment.
Conclusions
Long-term follow-up results obtained after GnRHa treatment indicated improvements in adult height. This treatment was largely reported to be effective, especially in patients who were diagnosed with CPP younger than 6 years of age and had received treatment, and GnRHa treatment did not seem to have a particularly adverse effect on reproductive function or bone growth.
However, proper treatment should be preceded by an accurate diagnosis and assessment of the ongoing disease process, as in some cases a normal adult height can be reached through the slow advancement of puberty without treatment. As mentioned above, the adequate GnRHa treatment dosage and systematic and consistent follow-up evaluation methods should be proposed in addition to an analysis of the cause behind the increase in CPP diagnoses and CPP treatment. A multicenter, large-scale research study of CPP patients in Korea is needed to obtain long-term follow-up data on the effects of CPP treatment on adult height, reproductive function, bone, and psychological aspects.
Notes
No potential conflict of interest relevant to this article was reported.