Nutritional strategy of early amino acid administration in very low birth weight infants
Article information
Abstract
Relative to a fetus of the same gestational age, very low birth weight (VLBW) infants are more likely to be underfed and to undergo growth restriction during their early hospital stay. The current trend towards "early and aggressive" nutritional strategies in VLBW infants aims to overcome the early nutritional deficiency and thereby boost postnatal catch-up growth, simultaneously improving long-term neurodevelopmental outcomes. Although the minimum starting amino acid (AA) dose to prevent negative nitrogen balance is well established, the upper limit and the rate of increase of early AA doses are controversial. Most randomized controlled trials show that early and high-dose (target, 3.5 to 4.9 g/kg/day) AA regimens, with or without high nonprotein calories, do not improve long-term growth and neurodevelopment. High-dose AA supplementation may lead to early metabolic disturbances and excessive or disproportionate plasma AA levels, particularly in infants of very low gestational age. Further large studies are needed to clarify the optimal strategy for early administration of parenteral AA doses in VLBW infants.
Introduction
Advances in parenteral nutrition (PN) have markedly improved survival rates of very low birth weight (VLBW) infants. However, a significant proportion of these infants still have growth restriction at hospital discharge1,2) and until adulthood3,4). Although both intrauterine growth restriction (IUGR) and postnatal growth restriction are well-known determinants of final growth outcome in preterm infants, postnatal growth restriction in particular, rather than IUGR status, is significantly associated with adverse neurodevelopmental outcomes during childhood5). Thus, whether "early and aggressive" nutritional support can normalize postnatal growth and simultaneously improve long-term neurodevelopmental outcome is one of the major interests in nutritional research of preterm infants.
Fetal nutrition has been regarded as the gold standard for postnatal nutritional therapy in preterm infants. According to the American Academy of Pediatrics' statement, the goal of postnatal nutrition in preterm infants is "to provide nutrition that will duplicate the in utero growth rate and body composition of the fetus at the same gestational age"6). However, this goal is difficult to achieve ex utero, particularly in the early days following birth. The recommended requirements for energy and protein intake, calculated by the factorial or empirical approaches, usually cannot be provided in VLBW infants because they are placed in highly humidified incubators and are frequently subjected to early fluid and nutritional restriction7,8). As enteral feeding is introduced, infants are gradually weaned from continual supplementation by PN, which mimics nutritional transfer from the placenta and would not be uninterrupted if they were in utero. Collectively, postnatal nutrition in VLBW infants cannot completely duplicate fetal nutrition quantitatively and qualitatively. This review will discuss early nutrition and the growth and developmental outcomes in VLBW infants, with a focus on amino acids (AA), the main building block of cell growth.
Early nutrition in VLBW infants: how does it differ from fetal nutrition?
The fetus needs a variety of substrates from the placenta for successful growth. Regarding the macronutrients, normal fetal nutrition can be briefly characterized as follows: AAs are transferred from the placenta at rates greater than that needed for net protein accretion within the fetus. The excess AAs are oxidized and contribute up to 20% of the fetus's total energy expenditure9). Glucose transfer is determined by maternal serum glucose concentration and occurs at rates that meet fetal requirements10). Lipids contribute minimally to the energy expenditure until the late part of the third trimester. Fat deposition increases markedly from the early third trimester and contributes to 10% to 15% of the fetus's body weight at term11,12). Current textbook recommendations for early PN in preterm infants advise higher lipid and lower protein intake than those required by the fetus13,14). Administration of intravenous lipid emulsion is usually recommended beginning at birth or postnatal day 1, and is encouraged to rapidly advance to 3.0 to 4.0 g/kg/day as long as hypertriglyceridemia does not develop. The short-term safety of a 20% lipid emulsion in preterm infants in terms of respiratory morbidities and metabolic abnormalities (such as hyperglycemia) has been confirmed, even during the early postnatal days15). However, reports of the higher adiposity and abnormal fat distribution in preterm infants relative to newborn infants at term equivalent age have raised concerns about these lipid-focused early nutritional strategies16,17). As for AA, factorial analysis reveals a decreasing requirement of AA as the fetus grows18) and a recent recommendation was provided for AA dosing to reflect this shift in fetal protein requirement19,20). However, neonatologists often increase the AA dose as the infant approaches hospital discharge because a significant proportion of preterm infants are considered to be behind their "healthy" peers (i.e., fetuses of the same gestational age) in terms of growth (Fig. 1).
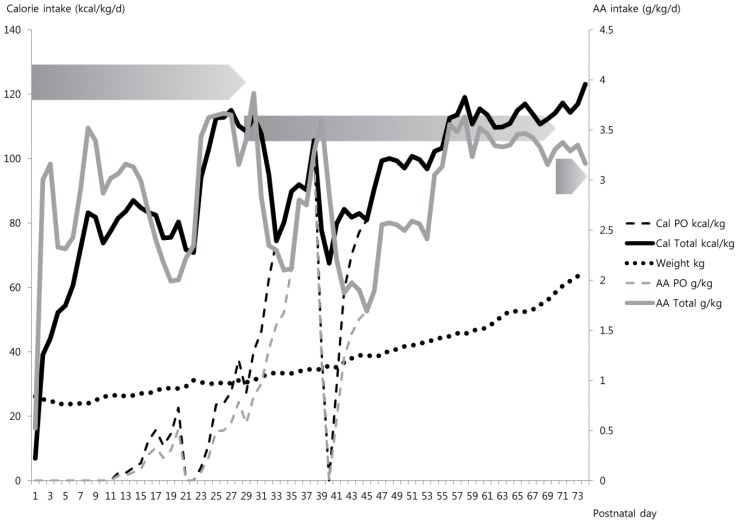
A sample nutritional plot of a female baby with a birth weight of 860 g born at 26+0 weeks gestation. An amino acid (AA) dose of 3.0 g/kg/day was started on the first day of life. Initiation of enteral feeding was delayed for 10 days after birth due to meconium plug syndrome and was withheld for symptomatic patent ductus arteriosus requiring surgical ligation (at 21-22 days) and transient sign of feeding intolerance at 40 days. Full enteral feeding was achieved with fortified breast milk at 32 days, but the recommended dose of AA could not be reached until 8 weeks after birth. Each graded pentagon indicates the recommended range of AA intake in preterm infants who do not require catch-up growth at 26 to 30 weeks, 30 to 36 weeks, and 36 to 40 weeks after conception, respectively (Data from De Curtis et al. Early Hum Dev 2012;88:S5-7.19)).
Optimal growth standard for very preterm infants
An intrauterine growth chart that represents overall fetal growth in a specific population is needed to assess the optimal postnatal nutritional care in preterm infants. Since the 1960s, numerous fetal growth curves have been developed from various subpopulations. Among these, Fenton's growth chart has been updated recently based on a meta-analysis of large population-based studies and is widely accepted for its high methodological quality21,22). A postnatal growth curve is another alternative to determine the growth standard for VLBW infants. However, if data are derived from preterm infants, "sick" survivors should be excluded to minimize the influence of postnatal conditions. Postnatal growth of individual preterm infants is determined by prenatal factors that caused preterm birth and by postnatal conditions, including various illnesses and nutritional practices23,24,25). However, data on the postnatal growth chart beyond the neonatal period are scarce. A growth reference chart derived from data of the Infant Health and Developmental Program (IHDP), which includes a large number of patients with longitudinally collected data from 40 weeks gestational age to 36 months, is recommended for VLBW infants requiring long-term follow-up26). However, the primary weakness of the IHDP growth chart is that the children analyzed were born in 1985 and likely had different medical and nutritional care than those of the current era.
The decision to adopt "fetal" or "postnatal" growth curve depends on the purpose for which it is used. The fetal growth curve is useful to compare VLBW infants with a normally growing fetus and may alert caregivers to increase calorie and/or protein supplementation to boost early catch-up growth. Meanwhile, the postnatal growth curve is useful to compare VLBW infants with their clinically stable peers as they receive "standard" nutritional care. Although this comparison may be reassuring to many caregivers, it may increase the proportion of preterm infants diagnosed with extrauterine growth restriction, at least by definition. Whether clinicians should promote early catch-up growth is a primary focus of nutritional studies of preterm infants with extrauterine growth restriction. Despite a potential benefit in neurodevelopmental outcomes5,27), the detrimental effect of rapid catch-up growth on cardiovascular and metabolic health in adulthood is an increasing concern, especially for infants born with relatively low birth weight or IUGR28,29). The rest of this article will summarize and discuss the results of recent randomized controlled trials that investigate the impact of early AA intervention on short- and long-term growth as well as neurodevelopmental outcomes in VLBW infants.
Optimal dose of early AA intake and metabolic influence
The most consistent finding in research on early nutrition of preterm infants is that increased protein intake produces greater gains in protein balance and that AA oxidation increases directly with supply30). To prevent negative nitrogen balance in VLBW infants, the recommended minimal starting dose of AA is 1.0 to 1.5 g/kg/day31). Below this, a loss of body protein is observed at a rate inversely related to gestational age. Protein loss in very preterm infants is approximately two folds greater than that in full-term newborn infants32). Prolonged deprivation of specific AAs, such as leucine, reduces insulin secretion and may lead to early hyperkalemia and hyperglycemia in newborn infants. However, early high-dose AA supplementation has not been shown to consistently prevent insulin-associated metabolic abnormalities better than standard doses33,34,35,36).
Although the lower limit of AA dose is generally confirmed among experts, whether the "fetal dose of AA" should be administered to VLBW infants in the early days of life is controversial. As noted earlier, the postnatal AA dose needed to maintain fetal protein accretion rate is at least 3.0 to 3.5 g/kg/day with an energy intake of more than 100 kcal/kg/day19,31,37). With respect to starting high AA dose from the early days of life, there has been concern about the risk of excessive products from AA metabolism. Fortunately, many comparative studies do not show a difference in the pH value or base deficit between groups taking different AA doses (the initial dose of AA ranged from 0 to 3.0 g/kg/day with a target range of 2.4 to 4.0 g/kg/day)33,34,38,39,40). Another concern regarding a high starting dose of AA is the risk of excessively high blood urea nitrogen (BUN) level. Several randomized controlled trials comparing high vs. standard doses of early AA doses show that patients taking high-dose AA have a significantly higher BUN than those taking standard-dose AA although the clinical implication of this difference is not clear. The suggested reference range for fetal BUN is 21.0 to 40.1 mg/dL34), but the safety limit in terms of brain development in very preterm infants is unknown. Whether elevated BUN level indicates AA intolerance and can be an indicator to withhold AA infusion remains controversial33,40,41,42). In practice, the AA infusion was frequently discontinued in infants with extremely low gestational age (≤24 weeks) particularly when accompanied by high ammonia levels33). Daily monitoring of individual AA level by comparing it with reference data can be a promising solution. Several types of automatic AA analyzers that use ion exchange chromatography or tandem mass spectrometry are available although their use is limited to research purposes41,42).
The influence of early AA intake on calcium and phosphorus homeostasis was reported recently in a study by Bonsante et al.43). Preterm infants (gestational age≤33 weeks) were divided into 3 groups according to their mean AA intake during the first week of life. The incidences of hypophosphatemia and hypercalcemia, respectively, were increased in the high (>2 g/kg/day of mean AA intake) AA intake group relative to the moderate (1.5-2 g/kg/day) and low (<1.5 g/kg/day) intake groups. This phenomenon is known as "Placental Incompletely Restored Feeding (PI-ReFeeding) syndrome". The proposed underlying mechanism behind this imbalance in serum calcium and phosphorus is that maintenance of anabolic status through parenteral AA and energy intake promotes cellular uptake of phosphorus, which leads to increased mobilization of calcium from the bones and hypercalcemia in the absence of adequate phosphorus intake. Although the clinical implication of this alteration remains unclear, it may increase the risk of bone mineralization defects in preterm infants44).
Early nutrition and neonatal morbidities
Does early nutritional strategy modify the course of critical illnesses in VLBW infants? Ehrenkranz et al.45) reported an association of the severity of illnesses with the early nutritional status. In a large study that analyzed the trial on parenteral glutamine supplementation performed by the Neonatal Research Network46), infants who were "less critically ill" received significantly greater amounts of parenteral and enteral nutrition during the first 3 weeks of life than infants who were classified as "more critically ill." Interestingly, those who were less critically ill also had a lower incidence of bronchopulmonary dysplasia (BPD), late-onset sepsis, and death, and a shorter duration of hospital stay, although the incidence of necrotizing enterocolitis was not different. However, the definition of "critically ill" in this study was somewhat arbitrary, as it was based on the duration of ventilator-dependency during the first 7 days of life. Additionally, the retrospective nature of this study also limits the ability to determine a causal relationship between early nutrition and neonatal morbidities. Most randomized trials fail to demonstrate a benefit of high-dose AA supplementation for reducing neonatal morbidities (see below for further discussion).
The role of nutrition for the prevention and management of BPD is of particular interest because malnutrition has a direct negative effect on the developing lung in humans and rats47,48). To date, no randomized controlled study has addressed the effect of early nutritional therapy on respiratory morbidities in preterm infants. In a limited number of prospective studies focused on growth and developmental outcomes, the incidence of BPD did not differ between those who received "early and/or higher AA intake" and those who received conventional timing and doses of AA49,50,51). Two studies have shown an association between the risk of BPD and delayed or lower quantity of enteral (but not parenteral) nutrition52,53). Because fluid intake is closely linked to calorie intake in the early neonatal period, it may confound the association between early nutrition and BPD. In a recent Cochrane Review, the fluid-restricted group tended to have a nonsignificant trend towards reduced incidence of BPD relative to the group with liberal fluid intake54). In another large retrospective study, high fluid intake during the first 10 days of life was associated with an increased risk of BPD in extremely low birth weight infants55). However, total fluid intakes reported in these early studies were considerably higher than those recommended by current protocols for VLBW infants typically managed in highly humidified incubators56).
Early parenteral amino acid doses and growth outcomes
One of the goals of early and aggressive PN is to minimize the initial growth deficit and maintain the fetal growth rate in each growth parameter. However, a benefit of early and high AA regimen (with or without an increase in overall calorie intake) on neonatal growth is not clear in most comparative randomized controlled trials51,57,58,59,60) (Table 1). Most studies show that growth parameters measured at 28 days of life and 36 weeks of corrected gestational age are not different between the early high-dose AA and conventional regimens. Only a recent study using a SCAMP (Standardized, Concentrated with Added Macronutrients Parenteral) nutrition regimen comprised of high doses of AA, glucose, and lipid, demonstrated greater head growth50) with a mean difference corresponding to 6% and 5% difference in brain weight at 28 days of life and 36 weeks of corrected gestational age, respectively. However, further research is necessary to determine whether the difference in head circumference can be maintained beyond infancy and translated into better neurodevelopmental outcomes.
Early parenteral amino acid doses and neurodevelopmental outcomes
The timing of nutritional deprivation is an important determinant of brain development. For example, preferential gene expression toward cell proliferation and differentiation in rats occurs around postnatal day 7, which is roughly equivalent to 32 to 36 weeks gestation in the human brain61). The human brain undergoes remarkable structural and functional changes between 24 to 42 weeks' gestation62), which implies that even a brief period of nutritional deficiency experienced during the early days of life can impair neurodevelopmental outcomes in VLBW infants.
A few clinical trials have investigated the relationship between the early AA intervention and long-term neurodevelopmental outcomes49,57,59). Despite common study designs with a target AA dose of 4.0 g/kg/day in the intervention groups, most studies failed to show better neurodevelopmental outcomes at various follow-up points. The study by Tan et al.59) was the only one to show that cumulative energy and AA deficits at 28 days correlated significantly with Bayley Scale of Infantile Development scores at 3 months in the entire cohort. However, this was not significant at 9 months postterm and the study did not show differences in the total or cortical brain volumes at 40 weeks' postconception. Similarly, Burattini et al.57) showed that an additional 8 g/kg of AA during the first 10 days of life did not improve the neurodevelopmental scores at 2 years of age. A series of studies by Blanco et al.33,49,63) showed that early and high-dose AA supplementation in extremely low birth weight infants was related to worse neurodevelopmental outcomes at 18 months of corrected age, though these were temporary. Although this analysis was limited by the high rate of loss to follow-up (about 50%), the potential toxicity of specific plasma AA on the developing brain in the high-dose AA group should not be overlooked63). In the studies that investigated the plasma AA levels after infusion of AA mixtures40,42,63,64), the study was designed so that the most premature infants received the highest doses of AA. As expected, the mean AA concentrations of the study by Blanco were greater than those in other studies. In addition, the specific essential (isoleucine, leucine, valine, phenylalanine, lysine, and methionine) and nonessential (proline) AAs were significantly higher in the intervention group than in the control group. In particular, leucine remained significantly higher for up to 7 days. Abnormal concentrations of branched-chain AAs have been related to impaired brain growth and development, possibly due to impairments with tissue protein synthesis/degradation, catabolism of other branched-chain AAs, and transport of large neutral AAs to the brain65). Studies comparing different doses and mixtures of AA supplements are needed to elucidate whether these differences are due to the high AA dose or the relative proportions of AAs in the mixture66).
Conclusion
Due to the high risk of growth failure and neurodevelopmental impairment in many VLBW infants, many neonatologists and nutritional experts suggest "early and aggressive" nutritional strategies with a primary goal of mimicking the AA dose that the fetus would receive in utero. However, the number of trials that compare standard and high-dose AA supplementation is limited and the benefits of such dosing on growth and developmental outcomes are unclear. The recent finding of an association between early high-dose supplementation and abnormal plasma AA levels, a protocol commonly used in extremely premature infants, indicates the need for an individualized approach to nutritional intervention. As for early parenteral AA nutrition, whether to follow the paradigm of "the more, the better" or "too much is as bad as too little" is still debatable. Further large studies are needed to clarify the safest and most effective strategy for early parenteral AA in VLBW infants.
Notes
No potential conflict of interest relevant to this article was reported.
