Approaches to the diagnosis and management of chronic urticaria in children
Article information
Abstract
Most guidelines for chronic urticaria (CU) in infants and children are based on limited pediatric evidence. Current evidence used to guide treatment in children is extrapolated from data focusing on older age groups. CU in children is a different and complex condition than that in adults. Furthermore, there is little published information regarding urticaria in Korean children. The aim of the present article is to review recent research on chronic childhood urticaria and improve the current understanding of its pathogenesis and management. The classification and definition of urticaria in adults also applies to children. CU is defined as a daily occurrence of spontaneous wheals, angioedema, or both for >6 weeks. The precise pathophysiology of CU is unknown and the rates of successful identification of a cause in children with CU vary from 20%-50%. There is no established laboratory test to evaluate the presence of urticaria. The natural course of childhood CU is undetermined, with limited reports discussing long-term outcomes. Second-generation H1 antihistamines are the cornerstone of management, while limited therapeutic drugs are available for adults.
Introduction
Urticaria, one of the most common skin diseases worldwide, is characterized by itchy wheals, angioedema, or both1). Although urticaria most commonly presents in children as a single episode lasting several days or weeks, many infants and children suffer from persistent urticaria. Chronic urticaria (CU) in children is a complex condition that differs from that in adults23). However, recommendations for managing children are based on extrapolating high-quality evidence from adults, as no pediatric data are available1). This review describes recent published recommendations for CU and information pertaining to chronic pediatric urticaria that may assist in its diagnosis and management.
The incidence of all forms of childhood urticaria is 3%-6%2). The prevalence of CU lasting >6 weeks is uncertain and varies among studies. The prevalence of CU in children in the UK is 0.1%-0.3%4). Among Spanish children under the age of 14 years with urticaria who visited the Emergency Department the preceding year, 18% were diagnosed with CU5). In Thailand, 13% of 142 children with urticaria were described as having CU6). In a recent study, no sex difference was found in children, unlike adults, where CU was found to be twice as frequent in female patients3789). In Korea, the median age of children with CU is 4 years89). There is no available information on the prevalence or differences in disease presentation according to age.
Definition and classification of urticaria and its severity
Urticaria manifests as wheals, angioedema, or both. A wheal is a central swelling of variable size, mostly surrounded by reflex erythema. It is accompanied by an itching or burning sensation and is transitory in nature. The skin returns to its normal condition within 2-24 hours after the appearance of symptoms. Angioedema is a sudden erythematous or skin-colored swelling of the lower dermis and subcutis, with frequent involvement below the mucous membrane, which may last up to three days1). Patients with angioedema typically experience more pain than itching. A prospective study indicated that 50%-60% of Thai children have wheals with angioedema7), while another study reported the presence of wheals alone in 78% of children, both wheals and angioedema in 15%, and angioedema alone in 6.6% of children10).
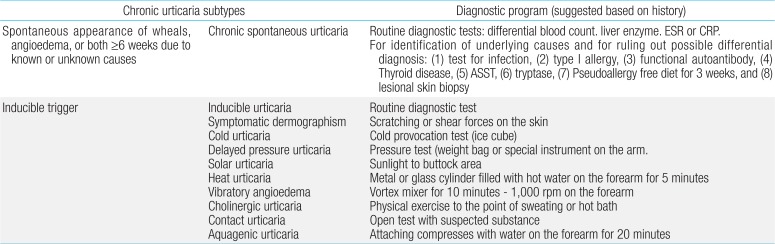
There are numerous overlapping classification systems for urticaria in children and adults. Urticaria is classified based on its duration and the presence of triggering factors. CU is defined as the occurrence of spontaneous wheals, angioedema, or both for >6 weeks. Spontaneous urticaria is considered in the absence of specific triggering factors. The term spontaneous urticaria has been proposed as a preferable alternative to idiopathic urticaria. The 2014 revised European and United States guidelines use the term inducible, indicating that it is triggered by a specific stimulus111) (Table 1).

Clinical classification of chronic urticaria subtypes (presenting with wheals, angioedema, or both) and recommended diagnostic tests
Wheals are a feature of other inflammatory diseases, including urticaria pigmentosa, urticarial vasculitis, auto-inflammatory syndrome (cryopyrin-associated periodic syndrome), and nonmast cell-mediated angioedema (hereditary angioedema and drug-induced angioedema). These diseases are not classified as subtypes of urticaria due to their different pathomechanisms. However, they should be considered in the differential diagnosis when a patient presents with urticarial manifestations111).
Spontaneous urticaria disease activity should be assessed by using the urticaria activity score 7 (UAS7), based on urticarial symptoms (wheals and itching). Overall disease activity is measured by advising patients to document 24-hour self-evaluation scores once daily for several days, due to frequent changes in the intensity of the condition. The UAS7 is the sum of the scores from seven consecutive days, facilitating measurement of disease activity and treatment response in routine clinical practice1) (Table 2).
Etiology
Many etiological factors have been associated with the onset of CU, but most cases are idiopathic. The rate of successful identification of a cause in children with CU varies from 20%-50%. Almost identified causes are inducible urticaria, which cholinergic, symptomatic dermographism, cold, and pressure urticaria are most common forms23). The following pathogenic conditions should be considered for spontaneous CU cases.
1. Infection
Infections have been suggested to play some role in causing urticaria in children12), as infections are identified in more than half of acute urticaria cases. Viral upper respiratory or digestive infections are the most frequent culprit. This contrasts with CU, where infection seems to be an exacerbating factor during the course of CU212). Although symptomatic or laboratory findings of Epstein-Barr virus, Mycoplasma, Chlamydia, or Helicobacter pylori infection have been detected in several children with CU, limited data prevented researchers from identifying a causal relationship with CU1012131415). The prevalence of serum H. pylori IgG was 54% in Korean children with CU, higher than that in the general population. However, five patients who experienced remission after eradicating H. pylori with medication relapsed to urticaria three months later8). It is believed that chronic or occult infection with parasites may play a role in urticaria. Few children with CU are infected with parasites, and antihelminthic medications do not induce a higher rate of CU remission than in patients with no parasites in their stool7). No clear association between infection and CU has been established.
2. Food, food additives, and drugs
An inhalant allergy is not considered a cause of CU. However, foods containing pan-allergens, such as plant pollen or seeds/fruits eaten on a regular basis, may be a cause of recurrent urticaria. An association between food and acute urticaria has been demonstrated, but its relationship with CU is controversial891617). Several studies have reported that ~10% of children with urticaria have a food allergy based on history and a positive IgE test. One study reported an association between food allergies and CU based on specific IgE, food history, and food challenges7). The results showed that 7 of 94 (7%) of CU patients had a confirmed food allergy. Of these, four patients experienced remission of symptoms after eliminating specific foods, such as shrimp or clams. Food allergies should be carefully evaluated as a cause of CU. Allergies to food additives are considered a pseudo-allergy rather than an allergic reaction and have been suggested as a rare cause of CU118).
Drugs may cause acute urticaria or CU in children. Antibiotics and nonsteroidal anti-inflammatory drugs (NSAIDs) may be culprits in CU, but these are prescribed during infections, making it difficult to ascertain actual causality11). However, one recent study suggesting a relationship between NSAID and CU, even in children, showed that a nonnegligible portion of children and adolescents with CU (10%-24% in CU patients) have an aspirin hypersensitivity19).
3. Autoimmune reactivity, autoimmune disease, and others
Autoreactivity related to CU has been a concern. An autologous serum skin test (ASST) is usually used to evaluate an autoimmune etiology, and has good sensitivity and agreement with histamine-release test results. Measurement of IgG autoantibodies toward IgE or its receptor (FcεR1α) on mast cells and basophils, as well as the basophil activation test, can generally be performed only in specialized laboratories. However, no correlation between IgG autoantibodies and ASST results has been reported20).
Autoreactive IgG against IgE or its receptor is associated with CU in 40%-50% of children. The ASST is positive in 35%-50% of children with CU72021). However, no differences in medication requirements or disease remission have been observed between children with negative versus positive ASST results. A total of 40% of 25 Korean children who received the test had a positive response to their serum9).
Several autoimmune diseases, including thyroid disease, rheumatoid arthritis, lupus erythematous, and celiac disease have been associated with urticarial episodes2). The prevalence of thyroid disease signs or symptoms was high in cases of CU in one study22), but markers associated with autoimmune diseases, such as antinuclear antibody (ANA) and erythrocyte sedimentation rate, showed no significant association. In two Korean studies of children with CU, no patients had abnormal thyroid dysfunction, but several patients had an elevated ANA titer89). Several case reports have suggested an association between CU and malignancy. If symptoms other than those from urticaria are recognized, further evaluations should be undertaken11).
Clinical features and prognosis
Wheals and angioedema are migratory and transient, resolving with no residual lesions in chronic spontaneous urticaria. Wheals in inducible urticaria are reproducibly induced by physical stimuli. They usually appear 10-20 minutes after provocation with adequate strength on suitable skin and disappear within 1 hour, except in delayed pressure urticaria. Patients with predominantly spontaneous urticaria may have one or more types of physical urticaria that may manifest themselves simultaneously4911).
Information on the natural course of CU is scarce, and the few studies in children show variable results. In a recent prospective study of chronic spontaneous urticaria, the remission rates at 1, 3, and 5 years from symptom onset were 18.5%, 54%, and 67.7%23), respectively. A retrospective study demonstrated a 1-year remission rate of 37%24). Positive ASST or ANA results do not determine the prognosis923). Sex and age have undetermined effects on but do not significantly affect the progress of CU. The prognosis for Korean children with spontaneous and inducible CU is better than in other studies mentioned previously, showing that a 1-year remission rate of 85% after the first visit to the hospital and a total symptom duration of 23 weeks (range, 6-100 weeks). ASST, ANA, sex, and age did not differ between the remission and non-remission groups89). Chronic physical urticaria in children has a longer and more severe course, with 12% of patients in symptomatic remission after one year. In 20 patients with remission, the duration of symptoms was 30 months, with more frequent symptom episodes related to a more prolonged course4).
Diagnostic approach and laboratory investigations
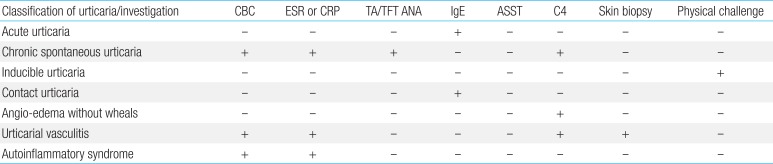
If clinical history and examinations indicate chronic inducible urticaria, further laboratory investigations are rarely useful in childhood. Targeted laboratory testing based on history or physical examination findings are appropriate, and limited laboratory testing should be conducted. These tests include examining liver enzymes and a complete blood count with differential, erythrocyte sedimentation rate, and/or C-reactive protein. If an autoimmune etiology is likely, measurement of ANA, complement, and thyroid function could be considered (Table 1). Limited laboratory testing might be appropriate to identify infrequent or rare cases where CU is caused by an underlying condition that might not be discernible based on history or physical examination findings or to provide reassurance to the patient and their family members1252627).
Food and food additives are undetermined causes of CU and should be excluded based on clinical history182628). Although allergy tests (skin prick tests and specific IgE tests) are useful in diagnosing IgE-mediated allergies, they cannot detect reactions due to food additives and dyes (non-IgE mediated allergies) or delayed immunological reactions2629). ANA should be measured only if a connective-tissue disorder is clinically suspected. A skin biopsy may be indicated if vasculitis is suspected. Hereditary or acquired deficiency of a C1 inhibitor has not been associated with urticaria. Therefore, further investigations are only indicated for children presenting with angioedema alone. Numerous autoimmune disorders, including systemic lupus erythematosus, dermatomyositis, and polymyositis, Sjögren's syndrome, and Still's disease have been associated with CU. However, serology to diagnose these underlying autoimmune diseases (such as connective tissue disease) is not warranted in an initial evaluation of CU in the absence of additional features suggesting concomitant autoimmune disease1252627). Serum cryoproteins are rarely found in children with cold urticaria. Investigations aimed at diagnosing current or past viral, bacterial, or parasitic infections should be guided by history, clinical findings, and initial screening tests. Limited data support the use of antiviral therapies in CU patients with concomitant herpetic infections or positive viral serologies1520) (Table 3).
Management
Second-generation H1 antihistamines are the cornerstone of management and avoidance of any identified provocateur is beneficial to reduce wheals and itching. NSAIDs, heat, and tight clothing can exacerbate CU in some patients. The utility of a pseudo-allergen-free diet for managing CU has not been convincingly demonstrated. Avoiding pseudo-allergens in the diet is not usually recommended11827). Potent topical corticosteroids may improve symptoms from delayed pressure urticaria but have limited utility for treating diffuse CU.
Evidence-based guidelines have been published on the diagnosis and treatment of CU12627). A step-wise approach has been developed for managing CU (Fig. 1). The mainstay of therapy is the use of second-generation H1 antihistamines. Cetirizine, ebastine, and loratadine are licensed in children ≥2 years of age. Levocetirizine can be given to children aged ≥1 year. Fexofenadine and azelastine are licensed for use in children >6 years old by the Ministry of Food and Drug Safety in Korea. Safety data are available for use of cetirizine in children 1-2 years of age at a dose of 0.25 mg/kg twice daily30). In patients that are nonresponsive to standard doses, pushing the H1 blockade with doses that are higher than the usual recommended doses of these agents is a common next approach, but data is limited and conflicting for some agents. Several treatment options can be used (step 2) for patients who do not respond to monotherapy with a second-generation antihistamine. Adding an H2 antagonist or antileukotriene agent can be considered for CU patients with an unsatisfactory response to second-generation antihistamine monotherapy. First-generation antihistamines can also be considered in patients who do not achieve control of their condition with higher-dose second-generation antihistamines. Although children may become accustomed to the sedating effects of first-generation antihistamines, there is a risk of psychomotor impairment, which may affect child safety and education. Treatment with hydroxyzine or doxepin, if not yet used, can be considered in patients whose symptoms remain poorly controlled, even after increasing the dosage of second-generation antihistamines and/or adding one or more of the following: an H2 antihistamine, a first-generation H1 antihistamine at bedtime, and/or an antileukotriene. Systemic corticosteroids are frequently used for patients with refractory CU, but no controlled studies have demonstrated efficacy. A short course of corticosteroids (for example, 1 mg/kg prednisolone twice/day, up to 40 mg total per day for 3 days) may be used for severe exacerbations. Short-term use of oral corticosteroids may be required to gain control until other therapies can control CU in children who remain poorly responsive to maximum doses of an H1 antihistamine, an H2 receptor blockade trial, or antileukotriene agent. Corticosteroids are not effective in patients with physical urticaria that are unresponsive to first-line therapy. Corticosteroids are more effective in patients with delayed pressure urticaria, but prolonged use results in unacceptable side-effects31). Since there is an increased risk of adverse effects with systemic corticosteroids, long-term use to treat patients with CU should be avoided as much as possible. Patients with CU whose symptoms are not adequately controlled by maximum antihistamine therapy (such as with step 3 care) may have refractory CU. A 4-6 mg/kg/day cyclosporine treatment has been effective in some adults with CU, but its use is limited by hypertension and/or nephrotoxicity. Some evidence from randomized controlled trials indicates the efficacy of cyclosporine2732). However, considering the limitations of those studies, the potential harm, cost of the treatment, and the low quality of evidence from these randomized controlled trials, there is a weak recommendation for cyclosporine use in patients with refractory CU27).
The therapeutic utility of omalizumab for refractory CU is supported by findings from large double-blind, randomized controlled trials, and is associated with a relatively low rate of clinically significant adverse effects3334). Omalizumab is approved for children ≥12 years by the U.S. Food and Drug Administration at both 150- and 300-mg doses for treating CU unresponsive to H1 antagonists. There is a lack of evidence for the efficacy of cyclosporine and omalizumab in children with CU. These drugs should be limited for use in difficult cases of children with CU and only considered in specialist centers. Many alternative therapies have been used in patients with refractory CU. Immunomodulatory agents have been used but their efficacy remain to be formally demonstrated. These drugs include hydroxychloroquine, ulfasalazine, colchicine, dapsone, mycophenylate, and intravenous immunoglobulin. Plasmapheresis has been used to treat autoimmune CU refractory to other therapies.
Conclusions
In this article, we have reviewed the available literature on childhood CU, including the limited reports on Korean children. CU is defined as the occurrence of spontaneous wheals, angioedema, or both for a period >6 weeks. Spontaneous urticaria is considered in the absence of specific triggering factors. The prevalence of CU in children is reported to be about 0.1%-0.3 %. Targeted laboratory testing based on history or physical examination findings is appropriate and might help in identifying the culprit. Second-generation H1 antihistamines are the cornerstone of management and avoiding any aggravating factors is beneficial to reduce wheals and itching. Information on the natural course of CU in children is scarce and the application of studies from adults to children is quite difficult due to the variations in disease. Two studies in Korean children are different from those outside the country in terms of laboratory findings and symptom duration. Clearly, there is a need for a nationwide study of the clinical presentation, laboratory investigation, and trends in CU management.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.