Cardiopulmonary function and scoliosis severity in idiopathic scoliosis children
Article information
Abstract
Purpose
Idiopathic scoliosis is a structural lateral curvature of the spine of unknown etiology. The relationship between degree of spine curvature and cardiopulmonary function has not yet been investigated. The purpose of this study was to determine the association between scoliosis and cardiopulmonary characteristics.
Methods
Ninety children who underwent preoperative pulmonary or cardiac evaluation at a single spine institution over 41 months were included. They were divided into the thoracic-dominant scoliosis (group A, n=78) and lumbar-dominant scoliosis (group B, n=12) groups. Scoliosis severity was evaluated using the Cobb method. In each group, relationships between Cobb angles and cardiopulmonary markers such as forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FEV1/FVC, left ventricular ejection fraction, pulmonary artery flow velocity, and tissue Doppler velocities (E/E', E'/A') were analyzed by correlation analysis linear regression.
Results
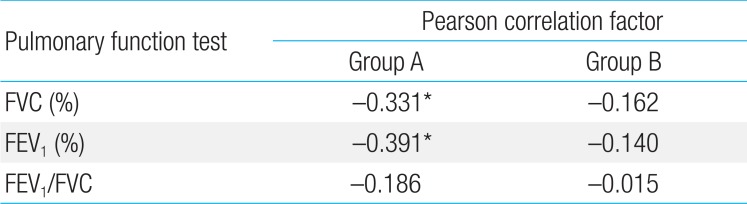
In group A, 72 patients (92.3%) underwent pulmonary function tests (PFTs), and 41 (52.6%) underwent echocardiography. In group B, 9 patients (75.0%) underwent PFT and 8 (66.7%) underwent echocardiography. Cobb angles showed a significant negative correlation with FVC and FEV1 in group A (both P<0.05), but no such correlation in group B, and a significant negative correlation with mitral E/A ratio (P<0.05) and tissue Doppler E'/A' (P<0.05) in group A, with a positive correlation with mitral E/A ratio (P<0.05) in group B.
Conclusion
Pulmonary and cardiac function was significantly correlated with the degree of scoliosis in patients with thoracic-dominant scoliosis. Myocardial diastolic function might be impaired in patients with the most severe scoliosis.
Introduction
According to the Scoliosis Research Society, scoliosis is defined as a lateral curvature of the spine greater than 10 degrees, as measured by the Cobb method on a standing radiograph1). For some patients, an underlying cause can be determined, including congenital changes, secondary deformities related to a neuropathic or myopathic condition, or degenerative spondylosis later in life. However, the cause of most scoliosis cases is not known, and such patients are diagnosed with idiopathic scoliosis2). Idiopathic scoliosis can be classified into three categories according to the age at which the deformity is detected: (1) infant: before three years of age; (2) juvenile: between the age of three and 10 years (or at the onset of puberty); and (3) adolescent: when it appears after 10 years of age or after the onset of puberty. Idiopathic scoliosis is one of the most frequent deformities involving the spine, with a worldwide incidence rate ranging from 0.5% to 10%3).
A 50-year-long follow-up cohort study of 332 untreated idiopathic patents in the United States was reported in 20034). There was no significant difference in estimated probability of survival between the patient group and the healthy population group. It was also reported that untreated idiopathic scoliosis causes little physical impairment other than back pain and cosmetic concerns4). Curves measuring form 30 to 50 degrees progress an average of 10 to 15 degrees over a lifetime. Curves greater than 50 degrees at maturity would progress steadily at a rate of 1 degree per year15). The curvature of a patient diagnosed with a curve over 50 degrees in the adolescent period will progress severely over their life compared to an individual with a less severe case of idiopathic scoliosis.
The thoracic spine is an important structure consisting of the thoracic cage in which major organs, such as the heart and lungs, are located. Structural changes of the thoracic cage can influence the functions of these vital organs, and several reports have identified a relationship between respiratory symptoms and scoliosis severity689). Also, structural anomalies of the heart, especially mitral valve prolapse, are frequently found in patients with scoliosis710). However, only a few studies have investigated the relationship between cardiac function and scoliosis severity61112).
In the current study, 90 patients who were diagnosed with idiopathic scoliosis and were undergoing correction of this disorder at a single institute (Spine center in Gangnam Severance Hospital) from September 2010 to January 2014 were retrospectively reviewed. Therefore, the purpose of this study was to find an association between preoperative cardiopulmonary function and scoliosis severity before surgery. We performed tissue Doppler imaging in addition to conventional echocardiograph measurements to evaluate diastolic and systolic myocardial function.
Materials and methods
1. Participants
We retrospectively reviewed the medical record of idiopathic scoliosis patients who had been diagnosed and followed at Gangnam Severance Hospital from September 2010 to January 2014. The patients who did not undergo both pulmonary function test (PFT) and echocardiographic evaluation before operation were excluded, and also patients with secondary scoliosis (congenital scoliosis, neurologic scoliosis, or muscular scoliosis) were excluded because their primary disease may affect their pulmonary and cardiac functions regardless of scoliosis itself. Total ninety patients were included in this study. Age at the time of surgery, sex, body surface area (BSA: m2), body weight (kg), and body mass index (BMI) (kg/m2) were recorded. All patients were divided into two groups according to whether the apex level in the curved spine was higher than the level of the diaphragm as seen in a standing radiograph.
2. Radiographic measurements
Standing, anterior-posterior radiography of the spine was performed preoperatively in all patients. To classify the severity of scoliosis, Cobb angle was obtained by a single investigator13). Fig. 1 demonstrates how Cobb angle was measured in patients with scoliosis. As scoliosis in the lumbar region has less of an effect on the thoracic cage structure compared to thoracic regional scoliosis, we tried to distinguish thoracic-dominant scoliosis from lumbar-dominant scoliosis. The two groups were defined as follows: group A, the apex of the scoliosis-affected spine was positioned above the diaphragm on the standing radiograph; and group B, the apex of the spine was below the level of the diaphragm. All idiopathic scoliosis patients were divided into one of these two groups.

The Cobb method of measuring the degree of scoliosis. The physician chooses the most tilted vertebrae above and below the apex of the curve. The angle between intersecting lines drawn perpendicular to the top of the superior vertebra and the bottom of the inferior vertebra is the Cobb angle (here, 62 degrees)1).
3. Pulmonary function test
Pulmonary function parameters were presented as the percentage of predicted values according to the patient's age, sex, height, and weight. Forced vital capacity (FVC) (%), forced expiratory volume in one second (FEV1) (%), and the FEV1/FVC ratio (%) were measured. Eighty-one out of the 90 patients (90%) underwent the PFTs. An FVC>70% and FEV1>70% were regarded as reasonable ranges.
4. Echocardiographic measurements
One pediatric cardiologist performed the preoperative thorough echocardiographic evaluations in all patients. The left ventricle ejection fraction was obtained with M-mode in the parasternal short axis view. The peak pulsed wave Doppler velocity in the main pulmonary artery flow was also evaluated in the parasternal short axis view. We procured the myocardial tissue Doppler velocities from the mitral lateral and septal annulus on the four-chamber view using tissue Doppler imaging in addition to conventional echocardiographic measurements including mitral inflow velocity (E/A). Among the 90 idiopathic scoliosis patients, 49 (54.4%) underwent echocardiography before corrective surgery for their scoliosis.
5. Statistical analysis
All data were analyzed with PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA) and presented as means±standard deviations. One-way analysis of variance was used to compare age, BSA, body weight, BMI, and Cobb angles between groups A and B. Correlation was analyzed between the Cobb angle and PFT (FVC, FEV1, and FEV1/FVC), and echocardiographic parameters (EF, E/A, E/E', E'/A') for each group, by obtaining the Pearson correlation coefficients. When statistical significance was achieved, subsequent linear regression analysis was performed. Statistical significance was defined as a P<0.05.
Results
The clinical demographic characteristics and the results of pulmonary function and echocardiographic measurements are listed in Table 1.

Comparison of clinical characteristics, pulmonary function test results, and echocardiographic findings between groups A and B
The male-to-female ratio was 15:75 (16.7% vs. 83.3%) in the 90 adolescent patients. The mean ages of the patients at the time of diagnosis were 14.8±2.2 years (range, 10.5-19.9 years). In total, 78 patients (86.7%) were classified into group A, and there were no significant differences in sex (12:66 vs. 3:9, P=0.405), age (177.1 months vs. 182.8 months, P=0.502), BSA (1.46 m2 vs. 1.52 m2, P=0.284), body weight (48.4 kg vs. 52.5 kg, P=0.227), BMI (19.0 kg/m2 vs. 20.3 kg/m2, P=0.207), or Cobb angle (53.8 degrees vs. 53.4 degrees, P=0.924) between the two groups (Table 1). None of the clinical characteristics were statistically correlated with Cobb angle in each group. In group A, 72 patients (92.3%) underwent the preoperative PFT, while in group B, 9 patients (75%) underwent the same tests. Only the FEV1 values (72.1% vs. 83.9%, P=0.003) were significantly different between the two groups. In groups A and B, preoperative echocardiographic examinations were performed in 41 cases (52.6%) and 8 cases (66.7%), respectively. There were no significant differences in the main pulmonary artery velocity (90.4 m/sec vs. 97.6 m/sec, P=0.658), left ventricular ejection fraction (66.9% vs. 67.8%, P=0.751), mitral E/A (2.07 vs. 2.41, P=0.090), tissue Doppler E/E' (6.40 vs. 6.65, P=0.644), or tissue Doppler E'/A' (2.11 vs. 1.91, P=0.435), between groups (Table 1).
1. Cobb angle and PFT
In group A, the mean FVC (%) was 79.3±18.4 (range, 28-130) of the predicted value, FEV1 (%) was 72.1±17.2 (range, 24-117), and the FEV1/FVC ratio was 85.9±6.9 (range, 57-97). There were significant negative correlations between Cobb angle and FVC, and Cobb angle and FEV1 in group A with Pearson correlation coefficients of -0.331 (P=0.004) and -0.391 (P=0.001), respectively. However, no significant correlation was found between FEV1/FVC and Cobb angle (Table 2). Preoperative FVC values were <70% of predicted values in 18 patients (25%), and FEV1 values were <70% in 29 patients (37%). In group B, none of the PFT parameters (FVC, FEV1, FEV1/FVC) were significantly correlated with Cobb angle (Table 2).
2. Cobb angle and echocardiographic parameters
In group A, significant negative correlations were found between Cobb angle and the mitral E/A ratio (Pearson correlation factor, -0.336; P=0.05) and tissue Doppler E'/A' values (Pearson correlation factor, -0.375; P=0.038). The echocardiographic parameters (main pulmonary artery flow velocity, left ventricular ejection fraction, mitral valve E/A ratio, and tissue Doppler E/E') showed no significant correlations with Cobb angles (Table 3). In group B, only conventional mitral E/A ratio was found to be significantly correlated with Cobb angle (Pearson correlation factor, 0.824; P=0.044) (Table 3).
Discussion
Scoliosis is the most common abnormality of the spine in adolescents14). The prevalence of adolescent idiopathic scoliosis using a Cobb angle cutoff point of 10 or more is approximately 2% to 2.5%2). Idiopathic scoliosis is generally more common in female children2). The ratio of girls to boys with low curvatures is equal but increases to a ratio of 5.4 girls to 1 boy for curves of 21 degrees or greater2) or 10 girls for every one boy with curves greater than 30 degrees1). In this study the overall range of Cobb angles was 28.5-94.0 degrees (53.8±15.0 degrees), of which, 83.3% were female patients. The ratio of girls to boys was about 5:1, which is comparable to previously published data.
As the scoliosis patients presented the lower compliance of the respiratory system, in particular the decreased chest wall compliance plays an important role in the impairment of lung volumes, and close correlation with the scoliosis severity and the decrease of FVC8). Scoliosis has generally been associated with the development of restrictive lung disease, which could result in decreased lung volume as manifested by a decrement of total lung capacity815). Cobb angle had a negative correlation with FVC and FEV1 values in group A, which implies that thoracic restriction associated with scoliosis decreases total lung capacity and subsequently FVC and FEV115). Expiratory flow rates are diminished in proportion to the restricted lung volume, while the FEV1/FVC ratio is within normal ranges8). Although the FEV1/FVC ratio is the most common measure of obstructive lung disease, there was no statistical correlation between FEV1/FVC and Cobb angle in the current study. This suggests that scoliosis patients do not develop obstructive lung disease. The proportions of patients with an FVC<70% and FEV1<70% were 25% (18) and 37% (29), respectively. The most scoliosis patient had normal PFT ranges, even though in severe scoliosis children showed the decreased PFT values with statistically significance. Linear regression analysis between FVC, FEV1, and Cobb angle in group A is shown in Fig. 2. Using the equations developed from the linear regression analysis, the Cobb angles were predicted to be 77.8 degrees and 59.0 degrees when the FVC and FEV1 were 70% respectively.
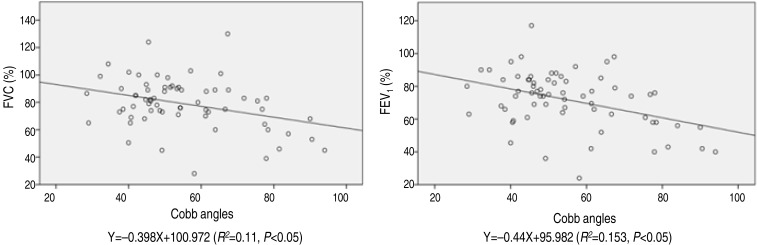
Linear regression analysis between Cobb angles and FVC and FEV1 in group A. Group A, the apex of the scoliosis-affected spine above the diaphragm; FVC, forced vital capacity; FEV1, forced expiratory volume in one second.
Thoracic-dominant scoliosis has an impact on the thoracic cage, which may change the pulmonary artery resistance or flow pattern. In this study, the exact measurement of pulmonary artery flow revealed a lower velocity in thoracic-dominant scoliosis (group A) compared to lumbar scoliosis (group B). This suggests that changes to the thoracic structure interrupt the laminar flow velocity in the main pulmonary artery. Although, the statistical data showed that the flow velocity in the main pulmonary artery was not significantly affected by the level of scoliosis, further study should be considered.
Tissue Doppler imaging in the longitudinal direction showed that the apex is generally immobile, whereas the base moves toward the apex in systole and away from the apex towards the annulus in diastole. This differential motion between base and apex results in a velocity gradient along the myocardial wall, with the highest velocities at the base and low or zero velocity at the apex1617). In patients with impaired diastolic cardiac function, the velocity of the E' wave decreased and the E'/A' ratio is usually below 117).
Distortion of the thoracic cavity caused by scoliosis could affect the subtle functions of the heart and large vessels by altering their position and their relation to each other15). Dhuper et al.10) reported that mitral valve prolapse is four times more common in patients with severe scoliosis than in the normal adolescent population. Li et al.11) have reported that tricuspid annular displacement decreases in patients with severe idiopathic scoliosis. Meanwhile, Liang et al.6) showed there were no significant differences in left ventricle ejection fraction and fractional shortening between patients with congenital scoliosis according to the Cobb angles. In the current study, the mitral E/A ratio and tissue Doppler E'/A' ratio in patients with thoracic-dominant scoliosis showed a significant negative correlation with the Cobb angle. Thoracic cage deformity could limit cardiac diastolic movement, as demonstrated in restrictive lung capacity of PFT, and also pulmonary venous return impairment may influence decreased early phase atrioventricular flow and/or atrial contraction. However, the other echocardiographic parameters, including the peak velocity of the main pulmonary artery, left ventricular ejection fraction, and tissue Doppler E/E' ratio, were not statistically correlated with Cobb angles in group A. Mostly, the Doppler measurement including tissue Doppler E/E' and E'/A' and left ventricular ejection fraction were within reasonable ranges in both groups (Table 1). When each patient's values were analyzed in detail, the tissue Doppler E/E' measurements were lower in the thoracic-dominant group compared to the lumbar-dominant group, as measured by the pulmonary artery flow velocity.
It has been reported that the estimated probability of survival of idiopathic scoliosis patients does not differ significantly from a matched general population group5). Furthermore, since it is difficult to define the precise relationship between cardiac functional change by echocardiography and scoliosis severity, further meticulous investigations should be performed to clarify the subtle cardiopulmonary functional changes in scoliosis patients.
Therefore, even though various parameters of the echocardiographic and Doppler examinations were within reasonable ranges, tissue Doppler E'/A' showed a negative correlation with Cobb angles in thoracic scoliosis, implying that scoliosis deteriorates diastolic myocardial function.
In conclusion, children with idiopathic scoliosis should undergo an evaluation of cardiopulmonary function regardless of symptoms, especially patients with larger Cobb angles. In the future, the idiopathic scoliosis children should be followed for their cardiopulmonary function after the spine correction operation.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.