Nutritional aspect of pediatric inflammatory bowel disease: its clinical importance
Article information
Abstract
Inflammatory bowel disease (IBD) is a chronic inflammatory disease mainly affecting the gastrointestinal tract. The incidence of the disease is rapidly increasing worldwide, and a number of patients are diagnosed during their childhood or adolescence. Aside from controlling the gastrointestinal symptoms, nutritional aspects such as growth, bone mineral density, anemia, micronutrient deficiency, hair loss, and diet should also be closely monitored and managed by the pediatric IBD team especially since the patients are in the development phase.
Introduction
Inflammatory bowel disease (IBD), including Crohn disease and ulcerative colitis, is a chronic relapsing inflammatory disease mainly affecting the gastrointestinal tract. It is believed to develop from an immune reaction that has been triggered by environmental factors in genetically susceptible individuals. Incidence of the disease is rapidly increasing worldwide and up to 25% of patients are diagnosed during their childhood or adolescence1). Although the exact incidence of IBD is not well known, it is also believed to be rapidly increasing in Korean children2). Only 4 patients were diagnosed with IBD from 1996-2001 in a single center study in Korea, while 58 patients were diagnosed with IBD from 2002-20073). Despite inclusion in the same disease category, IBD in early life appears to manifest in a different way compared to adult onset IBD. Since children and adolescents are in the process of maturation, this chronic inflammatory disease can adversely affect their growth and development. Therefore, additional concerns need to be addressed in pediatric IBD patients. In this review, we addressed some points for consideration in the management of pediatric IBD, which differs from adult IBD, especially with regards to the nutritional involvement.
Differences between pediatric IBD and adult IBD
As extensively studied by Ruel et al.4), there are several differences between pediatric-onset and adult- or elderly-onset IBD. With regards to epidemiology, Crohn disease is more prevalent than ulcerative colitis in pediatric-onset IBD. This is opposite to the findings in adult-onset IBD. Disease location and characteristics also differ between the two age groups. In the case of Crohn disease, proximal small bowel involvement and perianal disease are more frequent in children than in adults. In ulcerative colitis, extensive pancolitis is predominant in children, while proctitis or left-sided colitis is common in adult-onset disease. The natural course of the disease also differs according to the age of disease onset. In the case of Crohn disease, complications are more prevalent in early-onset compared to elderly-onset Crohn disease5) (Fig. 1). Similarly, in the case of ulcerative colitis, pediatric-onset disease has a more aggressive course compared to adult-onset ulcerative colitis4). The cumulative rate of colectomy at 5 years after diagnosis was 20% in pediatric ulcerative colitis, and only 8% in elderly-onset ulcerative colitis4). Given that IBD is a lifelong disease, early-onset patients will suffer for a longer duration and have a higher cumulative probability of complications.

The natural course of Inflammatory Bowel Disease accoring to age of disease onset. The disease phenotype was recorded according to the Montreal classification. CD, Crohn disease; B1, inflammatory phenotype without stricture formation and penetrating disease; B2, stricturing disease; B3, penetrating disease. Adapted from Gower-Rousseau et al. Dig Liver Dis 2013;45:89-94, with permission form Elsevier Ltd5).
Problems with growth and pubertal development
The majority of pediatric-onset IBD cases develop around pubertal age, which is a period of growth spurt and sexual development. The disease can disturb the normal development of pubertal changes, and affect the individual both physically as well as psychologically. Recent systematic reviews have shown that 10%-56% of Crohn disease and 0%-10% of ulcerative colitis patients had growth failure at the time of diagnosis6). One study involving Korean pediatric Crohn disease patients reported a 4% growth retardation rate at diagnosis, which is much lower than previous western studies. This might be due to ethnic differences, accessibility to gastrointestinal specialists or diagnosis timing in Korea7). Up to 46% of children with Crohn disease demonstrated lesser height increase before the onset of other symptoms, while only 12% demonstrated normal height at the time of diagnosis. Conversely, children with ulcerative colitis demonstrated a decrease in height velocity in only 3%-10% cases8).
According to a previous summary by Walters et al.9) on the pathophysiology of growth impairment in pediatric IBD, chronic caloric insufficiency is one of the greatest factors of growth deficiency. Reduced nutrient intake due to disease-related anorexia is a major factor and is believed to be related to tumor necrosis factor alpha (TNF-α) levels, a major inflammatory cytokine in IBD that interacts with the hypothalamic appetite pathway. Furthermore, unlike other nutritional deficiencies, the energy requirement for basal metabolism does not exhibit a compensatory reduction in IBD patients. Direct cytokines are also involved in this process. Insulin-like growth factor-1 is significantly reduced in spite of the normal growth hormone (GH) levels. Although the mechanisms are unclear, inflammatory cytokines such as TNF-α and interleukin-6 are believed to induce "GH resistance" in IBD patients. A frequently used medication for remission induction in IBD, corticosteroids can also aggravate growth deficiency in various ways. It can suppress central GH release and reduce hepatic GH transcription (Table 1). Genetic factors, such as NOD2/CARD15 polymorphisms, are believed to be factors of growth impairment9). Other problems involving the endocrine system, including a delay in pubertal development, are also frequently seen in Crohn disease. Previous studies have demonstrated that age at menarche and bone age are delayed in Crohn disease patients compared to the control group1011).
Although treatment priority remains focused on controlling disease activity and nutritional support, endocrinologic treatment, including the administration of recombinant human growth hormone, are showing positive preliminary results1112).
Decreased bone mineral density & osteoporosis
Patients with Crohn disease and ulcerative colitis are known to be at an increased risk of osteopenia and osteoporosis1314). Among adults with IBD, the prevalence of osteopenia and osteoporosis has been reported in 22%-77% and 12%-42% of patients, respectively15). One recent study reported that early (before the age of 30) disease onset is a risk factor for decreased bone mineral density16). In the pediatric population, significant deficits in the bone mass have been observed in 10%-40% of pediatric IBD patients. These are more prominent in patients with Crohn disease compared to those with ulcerative colitis 1517). Among Korean children, 20% of those with Crohn disease had bone mineral density z scores below-2 standard deviation at diagnosis7). Several factors are believed to be responsible for the pathogenesis of impaired bone mineralization in childhood IBD. These include prolonged corticosteroid treatment, low body weight, vitamin D deficiency, decreased physical activity, genetic problems, and inflammatory cytokines18). According to the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition, vitamin D status should be checked at least once a year in the springtime and supplementation considered17).
Anemia
Recent studies have demonstrated that anemia is the most common extraintestinal complication of IBD, affecting 19%-25% of patients19). Anemia is believed to reflect disease activity, and hematocrit levels are included in the pediatric Crohn disease activity index (PCDAI) score20). Chronic inflammation, as well as iron, vitamin B12 and folate deficiencies can cause anemia (Table 2)2122). Among these, iron deficiency anemia dominates more than half the cases (57%)23). Different types of IBD medications can also give rise to anemia. Iron deficiency is caused by chronic blood loss from the gastrointestinal tract as well as inadequate iron absorption from the duodenum due to mucosal inflammation and/or rapid bowel transit time. Additionally, ineffective iron utilization can also cause functional iron deficiency in IBD patients21). In cases of IBD patients with iron deficiency anemia, intravenous iron supplementation is the preferred treatment compared to oral iron therapy due to better tolerability and higher efficacy24).
Micronutrient deficiency
Patients with IBD are at a risk of micronutrient deficiency of vitamins and minerals. Decreased food intake, increased intestinal loss, intestinal, hypermetabolic state, drug interaction, and long-term parenteral nutrition, are believed to be associated with the pathogenesis of such deficiencies19). In the case of severe terminal ileitis or a history of ileal resection, there is also the risk of vitamin B12, folate, and fat-soluble vitamin deficiencies. Folic acid is not stored in the human body in large quantities, and even short durations of inadequate intake or malabsorption can cause folate deficiency, leading to megaloblastic anemia25). Folic acid deficiency can also be caused by sulfasalazine or methotrexate, which are inhibitors of dihydrofolate reductase and cellular uptake of folate 1926). Iron deficiency is also prevalent because of intestinal bleeding and ineffective utilization of stored iron due to proinflammatory stimuli. Zinc is an essential micronutrient for humans, and zinc deficiency is known to be common in patients with chronic diarrhea and malabsorption as well as those in a hypermetabolic state19). In one recent study, zinc deficiency was demonstrated in 40% of IBD subjects and 19% of the control population27). Since several micronutrient deficiencies are common in IBD, Hwang et al.19) recommend empirical supplementation and careful monitoring in special cases. For those undergoing sulfasalazine or methotrexate treatment, supplementation with 1 mg/day of folate is suggested. Those with significant diarrhea (>300 g/day) will need additional intake of magnesium and zinc. Patients with fistulas or nonhealing wounds need zinc and vitamin C while those with steatorrhea, multiple ileal resections, or severe ileitis require supplementation with fat-soluble vitamins and vitamin B12. Those patients on long-term total parenteral nutrition will need supplementation with iron, vitamin D, selenium, zinc, and manganese.
Hair loss
Although the exact prevalence and mechanisms are not well known, some patients complain about hair loss throughout the course of their disease. Autoimmune response, disease activity, malnutrition, micronutrient deficiency (such as zinc), anemia, and medications (such as methotrexate, azathioprine, and infliximab) are believed to be related to the increasing hair loss in IBD patients28). Although identification of the cause of hair loss in IBD is difficult, correction should be the first step in treating this condition. Since there are various types of alopecia, a dermatologist should be consulted for medical advice, especially in cases of focal alopecia, prolonged (6 months) hair loss, and if diagnosis remains uncertain29).
Exclusive enteral nutrition
Exclusive enteral nutrition (EEN) is recognized as an effective treatment strategy for pediatric Crohn disease. This therapeutic method is accomplished by supplying nutritional liquid formula without feeding the normal diet for several weeks. EEN is now recognized as a major form of therapy for pediatric Crohn disease patients and is recommended as a first line therapy for remission induction30). There is insufficient data on EEN among Korean patients. Although Suh et al.31) demonstrated the efficacy of EEN in Korean patients with Crohn disease, futher extensive long term studies are required. The mechanism of EEN is not exactly understood and several hypotheses have been considered. Gut rest and reduction of food antigens are believed to be one of these mechanisms. EEN is believed to modulate the intestinal microbiota in order to render it favorable for the noninflamed intestine. Several studies have demonstrated reductions in the proinflammatory cytokines and enhanced levels of anti-inflammatory proteins in patients treated with EEN32). Although EEN is a safe and effective treatment option, there is low acceptability due to exclusion of a normal diet. Partial enteral nutrition (PEN), which supplies liquid nutritional formula in addition to a normal diet, is ineffective for remission induction. Nevertheless, one report recently demonstrated the nutritional and clinical benefits of PEN33) (Fig. 2).
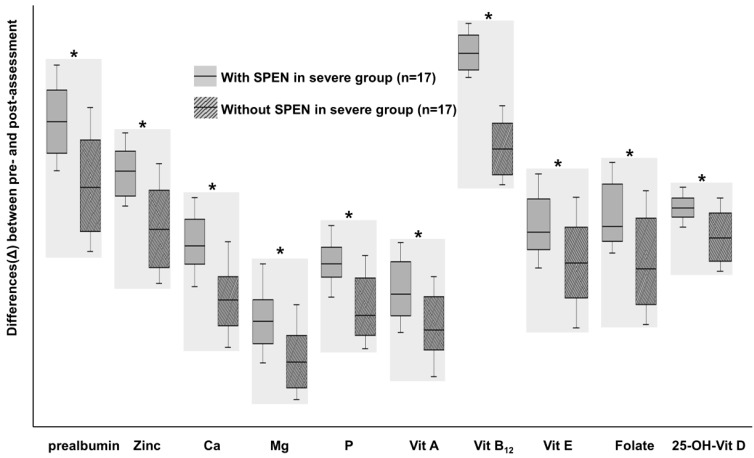
A comparison of the differences (Δ) in nutritional status among patients with severe Crohn disease at the initial enrollment and following 1 year of treatment between patients treated with and without supportive short-term partial enteral nutrition (SPEN), respectively. *P<0.05. SPEN were performed for 1 month after induction treatment. Micronutrients levels improved in both groups with larger increases observed in the SPEN group. Adapted from Kang Y, et al. Gut Liver 2015;9:87-93, according to open access policy of Gut and Liver33).
Dietary recommendations
Diet is believed to be a major contributory factor to the development and aggravation of gut inflammation through direct dietary antigens, alteration of the gut microbiome, and by affecting the gastrointestinal permeability34). Even if EEN is not implemented, some dietary modifications may induce a positive effect on the inflamed bowel. High dietary intake of total fats, PUFAs, omega-6 fatty acids, and meat are associated with an increased risk of IBD35). Diets low in fiber and residue are generally recommended for patients with Crohn disease in order to prevent bowel obstruction and reduce associated symptoms36). However, there is currently insufficient evidence of the effectiveness of such dietary modifications in IBD management. Since dietary habits vary between countries, nutritional interventions and dietary recommendations should be individualized.
Conclusions
The incidence of IBD is rising rapidly, with an increasing tendency for development of the disease among children and adolescents. Since the disease characteristics and natural history differs from those of adults, pediatric IBD patients should be considered differently, and the nutritional aspects of the disease should be prioritized appropriately.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.



