Low levels of tissue inhibitor of metalloproteinase-2 at birth may be associated with subsequent development of bronchopulmonary dysplasia in preterm infants
Article information
Abstract
Purpose
Bronchopulmonary dysplasia (BPD) is characterized by inflammation with proteolytic damage to the lung extracellular matrix. The results from previous studies are inconsistent regarding the role of proteinases and antiproteinases in the development of BPD. The aim of the present study was to investigate whether matrix metalloproteinase (MMP)-8, MMP-9, tissue inhibitor of metalloproteinase (TIMP)-2, and TIMP-1 levels in the serum of preterm infants at birth are related to the development of BPD.
Methods
Serum was collected from 62 preterm infants at birth and analyzed for MMP-8, MMP-9, TIMP-2, and TIMP-1 by using enzyme-linked immunosorbent assay. MMPs and TIMPs were compared in BPD (n=24) and no BPD groups (n=38). Clinical predictors of BPD (sex, birth weight, gestational age, etc.) were assessed for both groups. The association between predictors and outcome, BPD, was assessed by using multivariate logistic regression.
Results
Sex, birth weight, and mean gestational age were similar between the groups. BPD preterm infants had significantly lower TIMP-2 levels at birth compared with no BPD preterm infants (138.1±23.0 ng/mL vs. 171.8±44.1 ng/mL, P=0.027). No significant difference was observed in MMP-8, MMP-9, and TIMP-1 levels between the two groups. Multivariate logistic regression analysis indicated that the TIMP-2 levels were predictive of BPD after adjusting for sex, birth weight, gestational age, proteinuric preeclampsia, and intraventricular hemorrhage (β=-0.063, P=0.041).
Conclusion
Low TIMP-2 serum levels at birth may be associated with the subsequent development of BPD in preterm infants.
Introduction
In recent years, the quality of neonatal care in Korea has improved, resulting in a significant increase in the survival rate of neonates at high risk of mortality1). The improved survival of these infants has likely led to the increased incidence of a common corollary, bronchopulmonary dysplasia (BPD)2). BPD has long-lasting effects including poor neurodevelopmental outcomes and long-term pulmonary sequela. To date, there are few interventional therapies available to prevent or treat BPD3). Inflammation has been found to be central to the pathogenesis of BPD, but, unfortunately, this understanding has not yet translated into useful therapies.
The mechanisms underlying the pathogenesis of BPD have not been fully established, but proteolytic damage to the lungs from enzymes released by an influx of activated inflammatory cells is known to play a major role in the early stages. Furthermore, evidence suggests that proteolytic injury is expedited by an imbalance in lung proteinase versus antiproteinase defense456).
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that play an important role in the extracellular matrix remodeling that occurs during embryogenesis7), lung morphogenesis8), inflammation9), cardiovascular diseases10), metabolic disorders11), repair after injury12), and cancer13). A total of 25 MMP family members have been identified thus far, although not all are found in humans14). MMP-9 degrades collagen IV, a major component of the basement membrane of airways615). MMP-8 is another member of the MMP family that degrades type I collagen1617); it is associated with acute lung injury18192021) as well as chronic lung diseases such as asthma22) and BPD5). The activity of MMPs is controlled by specific tissue inhibitors of metalloproteinases (TIMPs)23). Previous studies have suggested that MMP-8, MMP-9, TIMP-2, TIMP-1, and their imbalanced ratios in bronchoalveolar lavage fluid (BALF) and blood may play a role in the pathogenesis of BPD, but the results of these studies have been inconsistent4562425262728).
The aims of the present study were to assess levels of MMP-8, MMP-9, TIMP-2, and TIMP-1 at birth in the serum of preterm infants and to determine whether these levels are associated with subsequent development of BPD.
Materials and methods
1. Study subjects
This study was prospective and enrolled preterm infants who were less than 32 weeks' gestation, had a birth weight of <1,500 g, required mechanical ventilation for respiratory failure, and were admitted into our neonatal intensive care unit between October 2010 and February 2012. Patients' and maternal characteristic data included sex, birth weight, gestational age as determined by the best obstetric estimate using ultrasound and/or the date of the mother's last menstrual period, the presence of proteinuric preeclampsia, the presence of premature rupture of membranes, chorioamnionitis, Apgar scores at 1 and 5 minutes, multiplicity, antenatal steroid used, the presence of respiratory distress syndrome (RDS), the number of surfactant replacement treatments, the duration of mechanical ventilation and oxygen supplementation, BPD disease severity (mild/moderate/severe), and development of patent ductus arteriosus (PDA), intraventricular hemorrhage (IVH)29), pulmonary hemorrhage30), necrotizing enterocolitis (NEC)31), and culture-proven sepsis. Mild BPD was defined as the need for supplemental oxygen (O2) for ≥28 days, but not at 36 weeks postmenstrual age (PMA) or discharge, moderate BPD was defined as the need for O2 for ≥28 days plus treatment with <30% O2 at 36 weeks PMA, and severe BPD was defined as the need for O2 for ≥28 days plus ≥30% O2 and/or positive pressure at 36 weeks PMA32). RDS was defined clinically. PDA was diagnosed via echocardiogram. The institutional review board of CHA University Gangnam Medical Center, Seoul, Korea reviewed and approved this study (IRB No. PKC10-009), and written informed consent was obtained from the parents of all infants.
2. Collection of blood samples
Blood collection was performed at the time of initial presentation immediately after delivery; samples were immediately centrifuged at 3,000 rpm for 5 minutes, aliquoted, and frozen at -20℃. The frozen serum samples were stored at -80℃ until use.
3. Determination of MMP-8, MMP-9, TIMP-2, and TIMP-1 levels
Serum concentrations of MMP-8, MMP-9, TIMP-2, and TIMP-1 were measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Briefly, a monoclonal coating antibody was adsorbed onto polystyrene microwell plates to bind MMPs or TIMPs in the samples or the standard. A horseradish peroxidase-conjugated monoclonal antibody with neutralizing activity toward MMPs or TIMPs was added to bind to MMPs or TIMPs captured by the first antibody. A substrate solution that was reactive towards horseradish peroxidase was then added to the wells to produce a colorimetric reaction in proportion to the amount of MMPs or TIMPs, and the absorbance was measured. The detection limits were 2.5 ng/mL for MMP-8 and MMP-9 and 2.4 ng/mL for TIMP-2 and TIMP-1. The MMP-8 and MMP-9 assay recognizes the proand active forms of MMP-8 and MMP-9.
4. Statistical analysis
Categorical data were expressed as number (percentage) and continuous data as median [interquartile range] unless otherwise indicated. Characteristics of the BPD and no BPD groups were compared using the chi-square tests or the Mann-Whitney U tests, as appropriate. The Mann-Whitney U test was performed to compare MMP-8, MMP-9, TIMP-2, and TIMP-1 levels between groups. Multivariate logistic regression analysis was used to assess the effect of TIMP-2, sex, birth weight, gestational age, proteinuric preeclampsia, and IVH on the risk of developing BPD, the outcome variable. All analyses were conducted using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). All statistical tests were two-sided and statistical significance was determined at a P value<0.05.
Results
1. Population characteristics and comparison of clinical parameters between preterm infants with and without BPD
The demographic and clinical characteristics and laboratory findings of the study population are presented in Table 1. Twenty-four preterm infants developed BPD and 38 did not. Of the 24 BPD infants, 16, six, and two infants developed mild, moderate, and severe BPD, respectively. The mean birth weights of the BPD and no BPD groups were 1,125.0±117.8 g and 1,181.8±121.4 g, respectively (P=0.923). The median gestational ages of BPD and no BPD subjects were 28+2 (26+3-31+6) weeks and 29+1 (26+2-31+6) weeks, respectively (P=0.875). BPD subjects were more likely to have mothers with proteinuric preeclampsia (16 [66.7%] vs. 10 [27.3%], P=0.036) and to develop IVH≥grade 3 (8 [33.3%] vs. 0 [0%], P=0.011). The BPD group had a longer mean duration of mechanical ventilation (21±13 days vs. 10±11 days, P=0.006) and oxygen supplementation (33±3 days vs. 17±18 days, P=0.002) compared with the no BPD group. There were no significant differences in sex, premature rupture of membranes, chorioamnionitis, Apgar scores at 1 and 5 minutes, multiplicity, antenatal steroid use, RDS, number of surfactant replacement treatments, or the incidence of PDA, pulmonary hemorrhage, NEC, or culture-proven sepsis between the two groups.
2. Comparison of MMPs, TIMPs, and their ratios between preterm infants with and without BPD
Preterm infants with BPD had significantly lower TIMP-2 levels at birth compared with those without BPD (138.1±23.0 ng/mL vs. 171.8±44.1 ng/mL, P=0.027) (Fig. 1C). No significant differences were observed in MMP-8 (2.0±2.2 ng/mL vs. 22.5±58.4 ng/mL, P=0.405) (Fig. 1A), MMP-9 (180.9±87.4 ng/mL vs. 285.4±533.7 ng/mL, P=0.165) (Fig. 1B), TIMP-1 (179.6±25.5 ng/mL vs. 176.8±44.2 ng/mL, P=0.692) (Fig. 1D) or their ratios between the two groups.
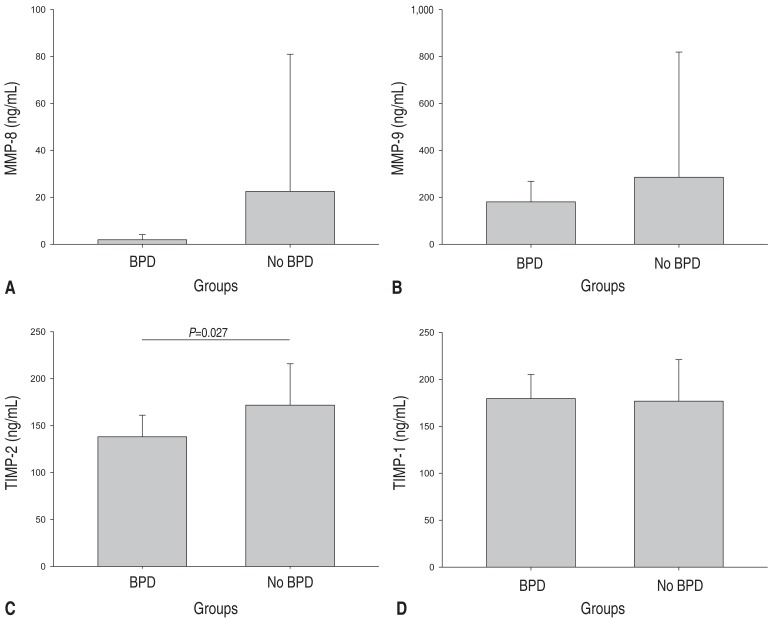
Serum concentrations of MMP-8 (A), MMP-9 (B), TIMP-2 (C), and TIMP-1 (D) at birth in bronchopulmonary dysplasia (BPD) versus no BPD preterm infants. Preterm infants who developed BPD had significantly lower levels of TIMP-2 at birth compared with those who did not develop BPD. MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase. Values are presented as mean±standard deviation.
3. Risk factors for the subsequent development of BPD in preterm infants
Multivariate logistic regression analysis was performed to evaluate independent relationships between the development of BPD and clinical and laboratory parameters. The results suggest that after adjusting for sex, birth weight, gestational age, proteinuric preeclampsia, and IVH, TIMP-2 level was significantly and inversely associated with subsequent development of BPD in preterm infants (β=-0.063, P=0.041) (Table 2).
Discussion
In this prospective study, we characterized the presence of MMP-8, MMP-9, TIMP-2, and TIMP-1 in the serum of preterm infants and found that a low level of TIMP-2 at birth in preterm infants may be associated with the subsequent development of BPD. To the best of our knowledge, the present study is the first to identify a significant association between low serum TIMP-2 concentration at birth and subsequent development of BPD in preterm infants.
The results of the present study correspond in part with those of previous studies. A study by Ekekezie et al.4) evaluated MMP-2, MMP-9, TIMP-2, and TIMP-1 levels in tracheal aspirate fluid samples obtained serially from birth until extubation in 49 ventilated preterm infants; they found low TIMP-1 levels and a higher MMP-9/TIMP-1 ratio during the first 2 weeks of life and low TIMP-2 and MMP-2 levels during the first 3 days of life in infants with BPD when compared to those without BPD. Another study by Cederqvist et al.5) analyzed tracheal aspirate fluids obtained within the first 5 postnatal days in newborns with RDS and observed lower TIMP-2 levels in those who experienced poor respiratory outcome (i.e., infants who developed BPD and those who died of severe respiratory distress) when compared to those who did not, but there were no observed associations between the development of BPD and MMP-2, MMP-9, or TIMP-2 levels. BALF is likely influenced by dilutional effects, however, and therefore MMP and TIMP measurements from BALF may not reflect the true physiologic status of newborns. The present study is unique in that MMPs and TIMPs were collected from the serum of preterm infants, which will not be affected by dilutional effects that are a major concern in studies assessing MMPs and TIMPs on BALF.
The mechanism underlying the relationship between low TIMP-2 level at birth in the serum of preterm infants and the development of BPD remains unclear. Possible underlying mechanisms for this association are as follows: First, TIMP-2 production depends on transcriptional regulation, thereby allowing for changes in the protein level to be tightly controlled. Hence, if TIMP-2 is not produced normally in response to regulatory signals, MMP-8 may degrade the extracellular matrix to a greater extent than it should. Second, TIMP-2 plays a unique role among TIMP family members in its capacity to suppress the proliferation of endothelial cells via metalloproteinase-independent mechanisms333435). Consequently, TIMP-2 may play an important role in maintaining tissue homeostasis by suppressing the proliferation of lung tissues in response to angiogenic factors, and by mediating protease activity in the lungs through extracellular matrix remodeling.
There are limitations to this study that need to be addressed. First, although we have data on total MMP-8 and MMP-9 immunoreactivity for all of the samples, we did not obtain data for MMP-8 and MMP-9 activity due to insufficient sample volume. The total level as determined from immunoreactivity consists of MMP that is fragmented, bound to TIMPs, or still in proenzyme form and thus does not reflect activity levels; however, other studies investigating MMPs, TIMPs, and their ratios as the basis for disease status have also based their analysis on these total values. Second, we did not obtain samples serially and therefore were not able to monitor changes in MMPs, TIMPs, or their ratios over time.
In conclusion, we described the presence of MMP-8, MMP-9, TIMP-2, and TIMP-1 at birth in the serum of preterm infants. Our findings indicate that there was a lower level of TIMP-2 at birth in the serum of preterm infants who subsequently developed BPD compared with those who did not. This finding suggests that a low serum TIMP-2 level at birth may play a role in the development of BPD in preterm infants by contributing to early lung inflammation. Thus, early postnatal supplementation of TIMPs could be an effective strategy in the treatment and prophylaxis of BPD. Further research is warranted to confirm this finding and to explore the possibility of TIMPs as a therapeutic and prophylactic option for BPD.
Acknowledgments
This study was supported by a 2010 research grant from the Korean Pediatric Society (Kyun-Il Award).
The authors would like to thank Miss Jin Ah Kim for her excellent technical support with data collection.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.



