Catch-up growth and catch-up fat in children born small for gestational age
Article information
Abstract
Infants born small for gestational age (SGA) are at increased risk of perinatal morbidity, persistent short stature, and metabolic alterations in later life. Recent studies have focused on the association between birth weight (BW) and later body composition. Some reports suggest that fetal nutrition, as reflected by BW, may have an inverse programing effect on abdominal adiposity later in life. This inverse association between BW and abdominal adiposity in adults may contribute to insulin resistance. Rapid weight gain during infancy in SGA children seemed to be associated with increased fat mass rather than lean mass. Early catch-up growth after SGA birth rather than SGA itself has been noted as a cardiovascular risk factor in later life. Children who are born SGA also have a predisposition to accumulation of fat mass, particularly intra-abdominal fat. It is not yet clear whether this predisposition is due to low BW itself, rapid postnatal catch-up growth, or a combination of both. In this report, we review the published literature on central fat accumulation and metabolic consequences of being SGA, as well as the currently popular research area of SGA, including growth aspects.
Introduction
Infants born small for gestational age (SGA) are at increased risk of perinatal morbidity, persistent short stature and metabolic alterations in later life1). Although approximately 70%–90% of SGA infants show catch-up growth during the first years of life, individuals born SGA may continue to have a short stature in adulthood23). The fetal origins hypothesis states that SGA children have a higher risk of developing metabolic syndrome (MetS) later in adult life4). There have been many recent reports of metabolic alterations in SGA children in later life, even in adolescence. In this report, we reviewed the published literature and the currently popular research area of SGA.
Definition and causes of SGA
The definition of SGA has been variably set at the 3rd or 10th percentile, or at less than –2 standard deviations (SDs) from the mean. Weight below the 10th percentile is used by neonatologists because it captures those at risk of perinatal morbidity and mortality5). The incidence of SGA births in each country is not exactly known, because birth anthropometric data and gestational age are rarely recorded in most national databases6). The prevalence of SGA (11.4%) in the 5th Korean National Health and Nutrition Examination Survey 2010–20117), conducted on Korean adolescents, is similar to that of other countries, including Japan, Norway, and the United States, using weight below the 10th percentile to define SGA8910).
Fetal growth depends on oxygen supply and blood vessel formation in the placenta as well as endocrine regulation of cellular expansion. The etiology of most SGA births remains unknown; however, several factors involving the fetus and placenta have been evaluated6111213). Maternal factors include poor nutrition, chronic disease and infections61314), as well as potential environmental toxins (e.g., smoking and alcohol consumption). Paternal factors including diabetes may also contribute to being born SGA15). Among these causes, lack of nutritional supply to the fetus is believed to be the primary cause of reduced fetal growth16).
Postnatal growth of SGA
Between 3% and 10% of all live neonates worldwide are born SGA. The majority of infants born SGA experience catch-up growth in the first few months, followed by a normal pattern of development. Catch-up growth of infants born SGA mainly occurs from 6 months to 2 years and approximately 85% of SGA children will have caught up by age 2 years2171819). SGA children are at high-risk of developing permanent short stature, and 10% continue to fall below the 3rd percentile of height into adulthood20). We reported significant positive relationships between birth weight (BW) for gestational age and the current height-standard deviation score (SDS) and weight-SDS in adolescents aged 10–18 years in Korea7). The growth hormone/insulin-like growth factor (GH/IGF) axis has a major role in promoting human fetal growth, as well as growth during infancy and childhood. Classic GH deficiency is rare in the SGA population6). Abnormalities in the GH/IGF axis have been reported in SGA children. Mean serum levels of IGF1 and IGF-binding protein-3 of SGA children at birth are known to be around 1 SD lower than for appropriate for gestational age (AGA) births. However, the serum levels of IGF1 of SGA children at later ages are contradictory. Some reports showed that SGA children have a higher IGF1 level than AGA children after catch-up growth2122). However, another report suggests a persistent lower IGF1 level in SGA children23). Recently, IGF1 gene deletions, point mutations, and polymorphisms have been described in populations born SGA2425). These long-term abnormalities of IGF1 in SGA may be implicated in the association with metabolic disease in later life222326).
General postnatal growth pattern can be divided into three phases: infancy, childhood, and puberty. Failure of growth in any of these phases can reduce growth potential and eventually cause adult short stature2728). SGA children who fail to catch up do not reach their target height range, and remain short throughout childhood and into adulthood2172930). The mechanisms of catch-up growth remain unclear. The finding of higher basal GH levels suggested hypersecretion as a factor in early catch-up growth31). On the other hand, BW, birth length, gestational age, and midparental height have also been identified as factors influencing catch-up growth171829).
Puberty in SGA tends to have a normal or slightly early onset3032), although age at menarche seems to be within the normal range32). Small variations from the normal pubertal growth pattern have been reported3334), but overall the final height prognosis in short children born SGA does not seem to be altered by the time of onset and/or progress of puberty293334). Being born SGA without adequate postnatal catch-up growth is a condition responsible for short stature in childhood and reduced adult height35); for adults with short stature, 22% were reported to be born SGA, if based on birth length2).
Insulin resistance and metabolic consequences of SGA
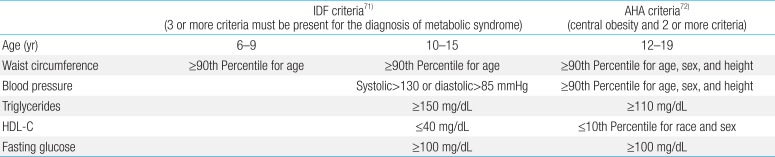
MetS is often referred to as the combination of central obesity, impaired glucose tolerance or overt type 2 diabetes mellitus, dyslipidemia, and hypertension36). For children, there are slight differences in the definition and basic criteria for MetS (Table 1)5). Excess visceral fat is strongly associated with free fatty acid (FFA) release and high FFA concentration can induce insulin resistance in muscle and the liver. Visceral adipose tissue is also prone to inflammation and inflammatory cytokine production, which contributes impairment in insulin signaling.
The "thrifty phenotype" hypothesis suggests that the fetus adapts to an adverse intrauterine environment giving rise to changes in insulin sensitivity and a predisposition to type 2 diabetes in later life4). The "fetal salvage" hypothesis also indicates the association between abnormal insulin sensitivity and a characteristic of subjects with intrauterine growth retardation37). In 1962, Neel38) suggested that genes promote survival and growth of the fetus in poor prenatal environments and induce the development of insulin resistance, given sufficient nutritional support after birth. Recently, several candidate genes have been regarded as contributing factors for developing insulin resistance39). Vu-Hong et al.40) showed the interaction between severe fetal growth restriction and the insulin gene variable number of tandem repeats locus, which were associated with insulin resistance in young adults born SGA.
Recent studies have also focused on the association between BW and later body composition (Table 2). Some reports suggest that fetal nutrition, as reflected by BW, may have an inverse programing effect on abdominal adiposity later in life. This inverse association between BW and abdominal adiposity in adults may contribute to insulin resistance. Byberg et al.41) reported that BW has a negative association with hypertension, insulin resistance and trunk fat in later life. Laitinen et al.42) suggest SGA itself is a risk factor for central obesity in female adults. Vaag et al.43) indicate that being born SGA and with low BW is associated with type 2 diabetes in a nongenetic manner, and programming of muscle insulin action and signaling represents an early mechanism responsible for this association. Rasmussen et al.44) report that low BW subjects had a significantly higher total abdominal fat mass and a higher proportion of trunk and abdominal fat mass but less leg fat relative to total fat mass. In spite of similar body mass index (BMI) and body composition, girls born SGA had a higher leptin level and insulinogenic index than AGA girls. These data suggest that low BW affects insulin resistance even in puberty45). Szalapska et al.46) observed a high frequency of MetS in Polish SGA children aged 5 to 9 years. The association of low BW was found to be significantly associated with such components of MetS as systolic blood pressure, diastolic blood pressure, triglycerides, insulin level, and insulin resistance, even in healthy Japanese high school girls47).
Children born large for gestational age (LGA) seem to have a larger body size but harmonic body composition and adequate body fat distribution. Children born SGA had higher central adiposity regardless of their body size48). Being SGA at birth could program excess abdominal fat deposition in children, which is a major component in the clustering of cardiovascular disease risk factors defining MetS.
Labayen et al.49) reported that impaired fetal growth, measured by BW, may be related to central fat distribution in Spanish boys. Labayen et al.36) also reported that adjusted BW z-score was inversely associated with central adiposity in male and female Spanish adolescents. Adjusted BW z-score was inversely associated with central adiposity, negatively associated with abdominal regional fat mass index independent of total fat mass, and inversely associated with the subscapular to triceps skinfolds ratio in boys5051). In 2014, American children with intrauterine growth restriction were reported to have higher waist circumference, higher insulin, higher homeostasis model assessment for insulin resistance (HOMA-IR), and lower adiponectin levels in adolescence, independent of other childhood and maternal factors52).
However many previous reports showed an inconsistent relationship between fat mass and BW. Choi et al.53) suggest that the association between low BW and insulin resistance is not mediated by abdominal obesity. Low BW was not associated with MetS in early adulthood8). Size at birth was positively associated with adult height and weight, but shows only weak association with BMI, and is not associated with waist/hip ratio when adjusted for socio-economic and lifestyle factors54). In data we previously reported, the prevalence of MetS was 1.2% and there were no differences in MetS components between SGA and AGA or LGA groups in 792 Korean adolescents7). Therefore, further studies are needed on the relationship between being born with low BW and metabolic risk.
Catch-up growth after SGA and central fat distribution in later life
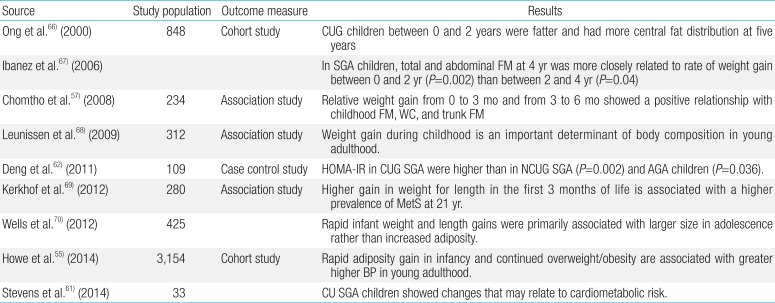
Rapid weight gain during SGA infancy seemed to be associated with increased fat mass rather than lean mass5556575859) (Table 3). Early catch-up growth after SGA birth rather than SGA itself has been noted as a cardiovascular risk factor in later life60).
Stevens et al.61) reported that catch-up SGA children are at high risk of cardiometabolic disease. Deng et al.62) report that HOMA-IR of term catch-up SGA children is higher than term AGA children. In a study on mice, forced catch-up growth after fetal protein restriction was reported to influence the adipose gene expression program63).
During recovery from wasting diseases and protein-energy malnutrition in children and adults, fat mass is accumulated much faster than muscle mass. These phenomena also occurred during SGA catch-up growth. Dulloo et al.64) noted that the insulin resistance seen in SGA catch-up growth is related to aforementioned phenomenon. This thrifty "catch-up fat phenotype" may be caused by complex interactions between earlier reprograming and a modern lifestyle characterized by nutritional abundance and low physical activity. The development of this catch-up fat phenotype is a central event that predisposes SGA children with catch-up growth to abdominal obesity, type 2 diabetes, and cardiovascular disease65).
Ong et al.66) showed that SGA children who showed catch-up growth between 0 and 2 years of age were fatter and had more central fat distribution at 5 years than other children. In SGA children, total and abdominal fat mass at 4 years was more closely related to the rate of weight gain between 0 and 2 years than between 2 and 4 years67). Leunissen et al.68) also report that weight gain during childhood is an important determinant of body composition in young adulthood, whereas birth size is less important. Kerkhof et al.69) demonstrates that a higher gain in weight for length in the first 3 months of life is associated with a higher prevalence of MetS at 21 years, whereas low BW is not. Wells et al.70) reported associations between rapid weight gain and fat mass in Brazilian adolescents.
Conclusions
SGA children have a tendency to accumulate intra-abdominal fat mass. It is not clear whether this is due to low BW itself, rapid postnatal catch-up growth, or a combination of both56). Catch-up growth has certain advantages in improved neurodevelopment, enhanced immune function, and final adult height. However, there are also certain disadvantages such as MetS, type 2 diabetes mellitus, cardiovascular disease, increased fat mass, and obesity. Therefore, early feeding of SGA children requires particular caution. In clinical practice, excess weight gain in SGA children should be prevented. Growth of SGA children should be measured every 3 months in the first year, and regular assessment of catch-up fat is necessary. Further studies are needed to prevent the complications of SGA as well as to develop and promote feeding guidelines for SGA children.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.