Bilateral iliac and popliteal arterial thrombosis in a child with focal segmental glomerulosclerosis
Article information
Abstract
Thromboembolic complications (TECs) are clinically important sequelae of nephrotic syndrome (NS). The incidence of TECs in children is approximately 2%–5%. The veins are the most commonly affected sites, particularly the deep veins in the legs, the inferior vena cava, the superior vena cava, and the renal veins. Arterial thrombosis, which is less common, typically occurs in the cerebral, pulmonary, and femoral arteries, and is associated with the use of steroids and diuretics. Popliteal artery thrombosis in children has been described in cases of traumatic dissection, osteochondroma, Mycoplasma pneumoniae infection, and fibromuscular dysplasia. We report of a 33-month-old girl with bilateral iliac and popliteal arterial thrombosis associated with steroid-resistant NS due to focal segmental glomerulosclerosis. Her treatment involved thrombectomy and intravenous heparinization, followed by oral warfarin for 8 months. Herein, we report a rare case of spontaneous iliac and popliteal arterial thrombosis in a young child with NS.
Introduction
Thromboembolism is a well-known complication of nephrotic syndrome (NS). However, in children, the incidence of thromboembolic complications (TECs) is relatively low, ranging from 2% to 5%1). Veins are most commonly affected, particularly the deep veins in the legs, the inferior vena cava, the superior vena cava, renal veins, and hepatic veins1). Dehydration, venepuncture, immobilization, and the use of central venous access devices, have been suggested as risk factors for TEC in children with NS23). Arterial thrombosis, which is less common, typically occurs in cerebral arteries, pulmonary arteries, and femoral arteries, and is associated with the use of steroids and diuretics4). Popliteal artery thrombosis in children has been described in traumatic dissection5), osteochondroma6), and fibromuscular dysplasia7). There is only one previous report of iatrogenic popliteal artery thrombosis with NS patient who underwent femoral puncture for blood sampling8). Here, we report a rare case of spontaneous iliac and popliteal arterial thrombosis in a young child with NS.
Case report
A 33-month-old girl presented with edema and proteinuria. Following clinical diagnosis of NS, she was treated with prednisolone (60 mg/m2/day) for 4 weeks, but NS has been persistent. When the patient was transferred to our hospital, physical examination revealed generalized edema, but no evidence of thromboembolism. Her vital signs were stable, with a blood pressure of 103/66 mmHg. Her weight was 12 kg (25th–50th percentile) and her height was 91 cm (25th–50th percentile). Laboratory studies revealed a white blood cell count of 8.38×109/L, hemoglobin level of 15.9 g/dL, hematocrit level of 46.1%, and platelet count of 284×109/L. Biochemistry showed the following: total protein, 5 g/dL; albumin 2.5 g/dL; total cholesterol, 235 g/dL; blood urea nitrogen, 11 mg/dL; creatinine, 0.3 mg/dL; calcium, 8.7 mg/dL; phosphorus, 5.7 mg/dL, and normal electrolyte level. Prothrombin time and activated partial thromboplastin time were both within normal range. Urinalysis revealed proteinuria of 4+ and the spot urine protein-to-creatinine ratio was 31.23 g/g Cr. Test results for antinuclear antibody and antineutrophil cytoplasmic antibody were negative, and the IgG, IgA, and IgM levels were 391.0 mg/dL, 82.0 mg/dL, and 142.0 mg/dL, respectively. Ultrasonography revealed normal-sized kidneys with diffuse increased renal parenchymal echogenicity. Renal biopsy revealed a collapsing variant of focal segmental glomerulosclerosis (FSGS) (Fig. 1).

Renal biopsy showing the collapsing variant of focal segmental glomerulosclerosis. Arrows indicate widened and effaced podocyte foot process. (A: Periodic acid-Schiff staining, ×400; B: electron microscopy image, ×2,500)
To manage the patient's steroid-resistant NS, high-dose intravenous methylprednisolone (MP) pulse therapy at a dose of 30 mg/kg was administered every 2 days. Angiotensin-converting enzyme inhibitor and intermittent albumin replacement followed by diuretics were also prescribed. After the first dose of MP, she complained of intermittent left lower leg pain, which was assumed to be a side effect of MP. After the third round of MP, her lower leg pain became continuous, progressed to both legs, and caused her to stop walking. The skin on the dorsum of her feet was pale and cold and capillary refill was slow. The dorsalis pedis pulse, posterior tibialis pulse, and popliteal pulse were palpable but not strong. Emergency Doppler ultrasonography of her lower extremities revealed turbulent flow in both her femoral and popliteal arteries. Magnetic resonance angiography showed multifocal segmental luminal occlusion of the common iliac arteries, external iliac arteries, and popliteal arteries (Fig. 2). The lumen of the common femoral arteries and superficial femoral arteries were relatively patent with distal flow reconstitution. The hemostasis study was as follows: thrombin time, 19.9 seconds (control, 17.0 seconds); fibrinogen, 362 mg/dL (reference, 180–380 mg/dL); D-dimer, 0.48 µg/mL (reference, 0–0.4 µg/mL); plasminogen activator, 86% (reference, 70%–140%); factor VIII, 214% (reference, 52%–190%); antithrombin (AT)-III activity, 91% (reference, 80%–120%); protein C, 243% (reference, 70%–140%); and protein S, 97% (reference, 65%–116%). Test results for anticardiolipin and anti-β2-glycoprotein-I antibodies were all negative. The factor V Leiden (1691G>A) mutation was absent.
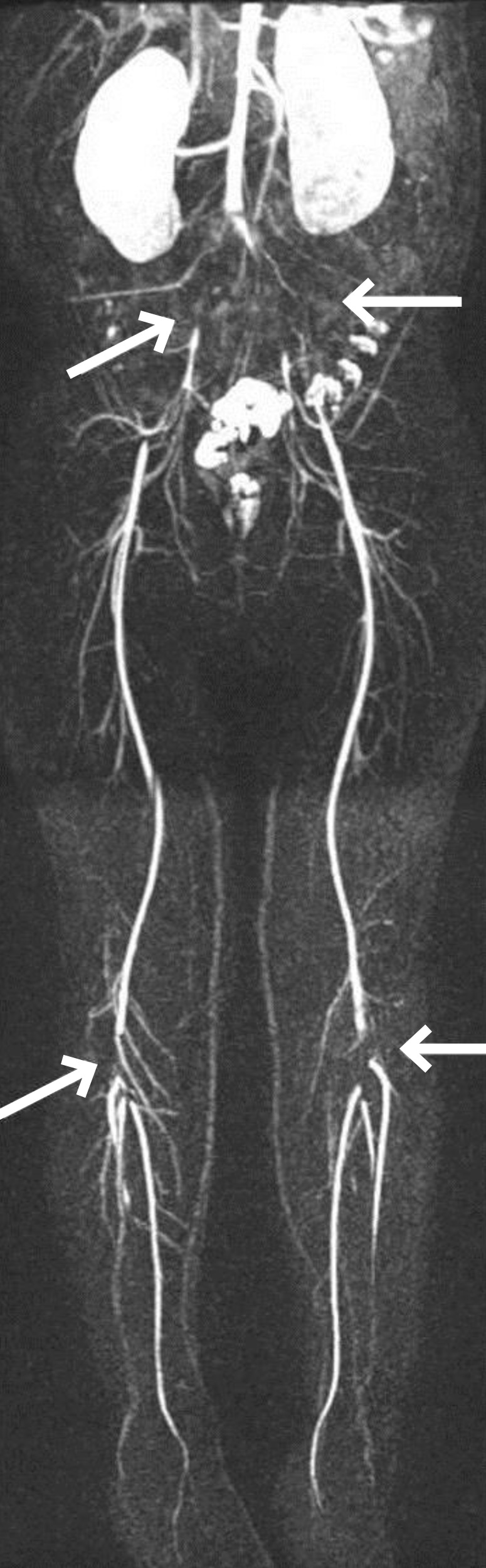
Magnetic resonance angiography showing multifocal segmental luminal occlusion at both the common iliac arteries, the external iliac arteries, and the popliteal arteries, indicated with arrows.
TEC was suspected, and MP pulse therapy was discontinued and heparinization started, followed by mechanical thrombectomy of both iliac arteries and popliteal arteries using Forgarty balloon catheters. Thrombus cultures were obtained during mechanical thrombectomy despite the absence of clinical infection. Staphylococcus hominis subspecies hominis (S. hominis subsp. hominis) was isolated, which was found to be ampicillin-resistant and vancomycin-susceptible. Since peripheral blood culture results were negative, systemic antibiotics were not administered. Anticoagulant treatment was continued for 8 months with warfarin. Two months later, a follow-up Doppler ultrasonography of her lower extremities showed patent lumen and intact flow patterns in the common femoral arteries, superficial femoral arteries, and popliteal arteries. The severity of proteinuria, which was consistent with NS, persisted despite various combinations of oral steroids, cyclosporine A, tacrolimus, and rituximab. The patient's renal function deteriorated and progressed to end-stage renal failure, and renal replacement therapy of peritoneal dialysis was started 22 months after TEC onset.
Discussion
Although TEC is a well-known clinically important complication of NS, identifying TECs in children is not easy because of its rarity in this population, particularly that involving the arteries. Here, we present a case of TEC involving the arteries that eventually resolved, despite a somewhat delayed diagnosis. Difficulty in obtaining a clear description of symptoms because our patient was young further hindered prompt diagnosis of TEC.
According to the literature, TECs often develops within months of diagnosis and during treatment of NS. The independent risk factors for TECs in children with NS are the severity of proteinuria and older age (>12 years)3). Dehydration, infections, venepuncture, and immobilization also predispose individuals to TECs2). TECs in our patient also occurred within 2 months of diagnosis and during treatment of NS, although she was only 33 months old. A week before diagnosis of TECs, the patient was placed under complete bed rest for 1 day after a renal biopsy. She might have been dehydrated for some time during this period, since her initial hemoglobin level was relatively high, and she needed several doses of albumin replacement therapy for hypovolemic symptoms and abdominal pain.
In adult NS, hypercoagulability develops due to urinary loss of the factors that participate in thrombosis and thrombolysis, especially vitamin K-dependent anticoagulating factors, protein C, protein S, and AT III1). Interestingly, in our case, assays of these proteins showed normal or increased levels of protein C/S and AT III, with an increase in the protein C level by 243%. Such a raised level of anticoagulating factors in pediatric NS was not a new finding, according to our literature search9). It is unknown if the raised level of protein C was secondary to the activation of immune system changes, as suggested by Neyrinck et al.10), or a compensatory activation against the TEC event. Steroid treatment is also thrombogenic, as it enhances hepatic synthesis of intrinsic and extrinsic coagulation factors and reduces plasma fibrinolysis11).
TEC is not common in children with NS, especially arterial TEC. The reason for this complication in our patient is debatable; the question of whether the type of NS increased her predisposition to TECs compared with idiopathic pediatric NS needs to be addressed. While Lilova et al.2) reported that the frequency of TECs in steroid-resistant NS was 2 folds or higher than that in steroid-sensitive NS, others reported otherwise4). With regard to pathologic diagnosis, Kerlin et al.3) reported that children with secondary NS or membranous nephropathy were more prone to TEC than patients with other pathologic diagnoses. Although her NS was presumed to be primary because we could not identify other causes, the patient had a collapsing variant of FSGS, which is common in secondary NS. Therefore, her disease course may resemble secondary NS in terms of complications as well as massive proteinuria and rapid progression to chronic kidney disease.
The presence of arterial TEC in our patient is interesting. TECs more commonly occur in veins than in arteries, unless other thrombogenic diatheses are involved, such as protein C/S deficiency, AT III deficiency, or antiphospholipid syndrome4). The most common sites of arterial thrombosis are the cerebral, pulmonary, and femoral arteries49), where turbulent flow occurs from arterial branching12). In patients with NS, arterial thrombosis typically occurs in the peripheral arteries in association with infection or arterial punctures, inducing stasis in blood flow4). However, our patient had TEC in her popliteal and iliac arteries, but not in her peripheral arteries. Popliteal artery thrombosis is universally associated with traumatic vascular injuries, tibial fracture, or osteochondroma located near the knee joint56). It is unlikely, however, that the patient had pre-existing injury in these vessels because she did not have a history of femoral puncture or other injuries, other than a renal biopsy. Interestingly, studies on thrombus cultures indicated infection with S. hominis subsp. hominis, which is known to be found on human skin and to cause infection in immunocompromised hosts13). The patient may have developed transient bacteremia as a result of venepuncture or renal biopsy. In addition, her severe NS status, prolonged dehydration, placement under absolute bed rest after renal biopsy, and high doses of the steroid MP, might have worked in concert, resulting in TECs.
With regard to the treatment of TECs in children, the American College of Chest Physicians, recommends intravenous unfractionated heparin (UFH) as the initial treatment for pediatric patients with a femoral artery thrombosis, and suggests thrombolytic therapy and surgical intervention as rescue therapy14). Intravenous UFH was not effective in this case, and thrombolytic therapy was not indicated due to delayed diagnosis15); therefore, surgery was performed with satisfactory outcomes. We do not know whether the resolution of arterial TEC in our patient's case is exceptional, given the limited data on arterial TEC in children with NS. In general, the response to TEC therapy in childhood NS is good, with complete recovery expected in >90% of cases, in which heparin treatment is followed by oral anticoagulants. On the other hand, pulmonary thromboembolisms can be fatal24).
In this case of massive arterial TEC in steroid-resistant NS, severe proteinuria, steroid therapy, chronic dehydration, and placement under absolute bed rest after renal biopsy, may have all contributed to the development of TECs. Thus, we suggest that vigilance against TECs is necessary in refractory NS, since any level of TEC may be possible and may require aggressive management.
Acknowledgment
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A120017). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2012R1A1A2006858 ).
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.