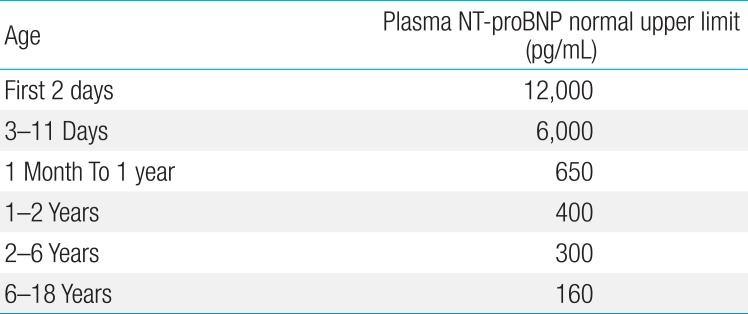
Age-adjusted plasma N-terminal pro-brain natriuretic peptide level in Kawasaki disease
Article information
Abstract
Purpose
Recent reports showed that plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) could be a useful biomarker of intravenous immunoglobulin (IVIG) unresponsiveness and coronary artery lesion (CAL) development in Kawasaki disease (KD). The levels of these peptides are critically influenced by age; hence, the normal range and upper limits for infants and children are different. We performed an age-adjusted analysis of plasma NT-proBNP level to validate its clinical use in the diagnosis of KD.
Methods
The data of 131 patients with KD were retrospectively analyzed. The patients were divided into 2 groups—group I (high NT-proBNP group) and group II (normal NT-proBNP group)—comprising patients with NT-proBNP concentrations higher and lower than the 95th percentile of the reference value, respectively. We compared the laboratory data, responsiveness to IVIG, and the risk of CAL in both groups.
Results
Group I showed significantly higher white blood cell count, absolute neutrophil count, C-reactive protein level, aspartate aminotransferase level, and troponin-I level than group II (P<0.05). The risk of CAL was also significantly higher in group I (odds ratio, 5.78; P=0.012). IVIG unresponsiveness in group I was three times that in group II (odds ratio, 3.35; P= 0.005).
Conclusion
Age-adjusted analysis of plasma NT-proBNP level could be helpful in predicting IVIG unresponsiveness and risk of CAL development in patients with KD.
Introduction
Kawasaki disease (KD) is an acute systemic vasculitis that occurs in infants and young children, and is important cause of acquired heart disease in developed countries1). Recently, plasma N-terminal fragment of B-type natriuretic peptide (NT-proBNP) level has been used as a clinical biomarker in KD23). Many studies revealed that NT-proBNP was helpful in predicting intravenous immunoglobulin (IVIG) unresponsiveness or the development of CAL45), and various cutoff values have been proposed. However, the serum concentration of this peptide vary according to patient age, with its normal range in infants and young children being different from that of adults6). So we investigated whether age-adjusted interpretation of plasma NT-proBNP level might be needed or be useful.
Materials and methods
1. Patients and materials
We performed retrospective review of the medical records of 131 patients who were hospitalized for KD at Konyang University Hospital between May 2013 and December 2014. The diagnosis of KD was established by use of American Heart Association guidline7). In our center, all the KD patients were treated as follows; IVIG (2 g/kg/day) infusion was done over 10–12 hours with oral aspirin. Laboratory tests were during acute phase and 48–72 hours after IVIG treatment. The echocardiography was performed by a pediatric cardiologist to document CAL in acute phase and 8 weeks later. CAL was defined as a coronary artery internal dimeter with a z score of ≥2.5 in at least one of the following coronary arteries: right, left anterior descending, and left main8).
The patients were divided into 2 groups as either higher or lower than the 95th percentile of NT-proBNP concentrations by use of reference value reported in the literature6). Group I was composed of patients with plasma NT-proBNP values within the 95th percentile, whereas group II was composed of those with plasma NT-proBNP values within the ≤95th percentile (Table 1). The clinical data and laboratory data including complete blood count; erythrocyte sedimentation rate; serum levels of C-reactive protein (CRP), electrolyte, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total protein, albumin, creatine phosphokinase-MB, troponin-I, and NT-proBNP were analyzed. We also compared the responsiveness to IVIG and the risk of CAL in both groups.
2. Statistical analysis
All of the data were analyzed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). Clinical characteristics and laboratory findings were statistically analyzed via a t test, with a P value of <0.05 defined as being statistically significant. The Spearman correlation analysis was done for elucidate the relation age and plasma NT-proBNP level. We performed logistic regression analysis to yield an odds ratio (OR) that approximates the relative risk of CAL according to plasma NT-proBNP level. The Pearson chi-square analysis was used in determining whether CAL and IVIG responsiveness differed between groups I and II.
Results
1. Demographic and clinical findings
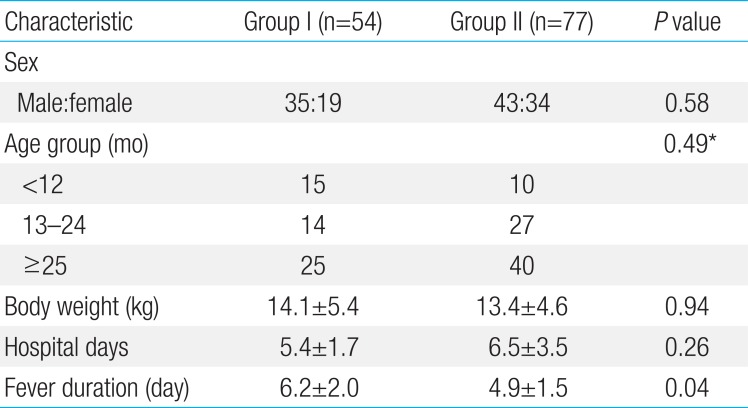
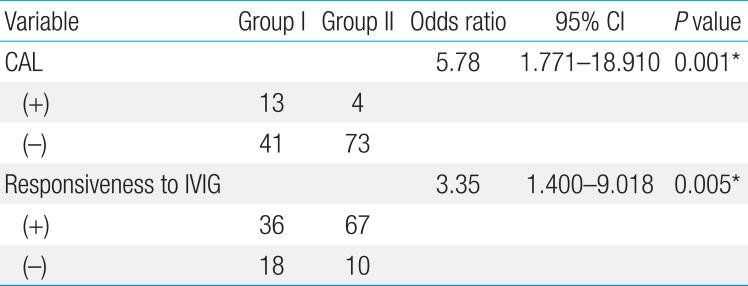
One hundred thirty-one patients with KD were enrolled in our study. Male outnumbered female by 78:53. The mean duration of fever of the patients with KD before admission was 5.1±1.8 days. The total hospital days was 5.6±2.5 days. The range of age of patients was 3–92 months (mean±standard deviation, 33.1± 23.6), 25 patients (19.1%) were infancy, 41 (31.3%) were 13–24 months of age, and 65 of all KD patients (49.6%) were older than 25 months. As for plasma NT-proBNP level, the younger age of patient with KD was, the higher level of this peptide was (r2=0.457, P=0.001) (Fig. 1).
Plasma NT-pro BNP level in patients with Kawasaki disease was inversely correlated with age (r2=0.457, P=0.001). NT-proBNP, N-terminal pro-brain natriuretic peptide.
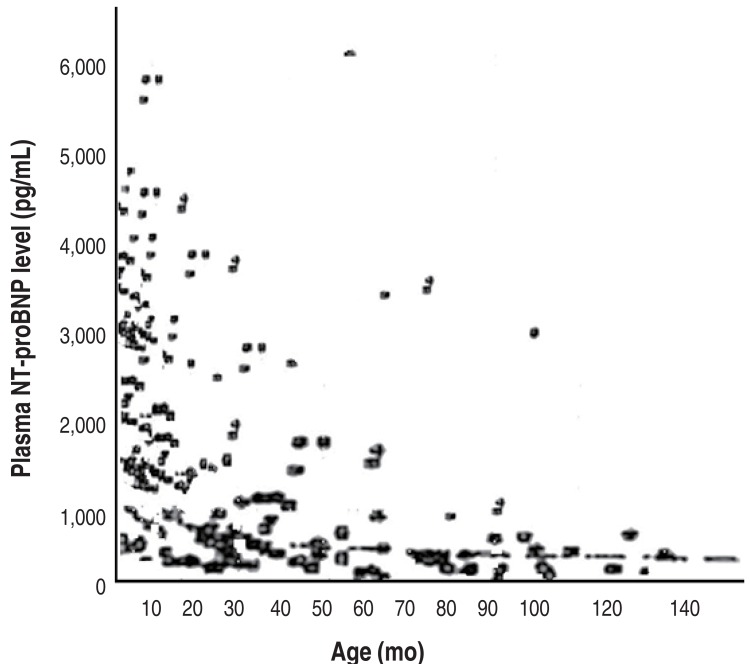
Among the 131 KD patients, CAL was discovered in 17 (12.9%). The CAL incidence was 8 (32%), 4 (9.7%) and 5 (7.6%) in each age groups, respectively (P=0.007). Plasma NT-proBNP level was notably higher in the group with CAL than in the group without CAL (1,931±1,914 pg/mL vs. 982±746 pg/mL, P=0.001) (Fig. 2).
Serum NT-proBNP levels in Kawasaki disease (KD) patients with and without coronary artery lesions (CALs). Serum NT-proBNP levels in KD patients with CAL (+) were 1,931±1,914 pg/mL and in KD patients without CAL (–) was 982±746 pg/mL (P<0.05). NT-proBNP, N-terminal pro-brain natriuretic peptide.
Twenty-eight patients (21.4%) did not respond to the first IVIG therapy, thus additional IVIG infusion and immunosuppressive therapy such as methylprednisolone were performed. But the plasma NT-proBNP level was not statistically different in both IVIG-unresponsive and IVIG-responsive group, (1,838±2,294 pg/mL vs. 906±1,835 pg/mL, P=0.051).
2. Relative risk for of CAL and IVIG responsiveness
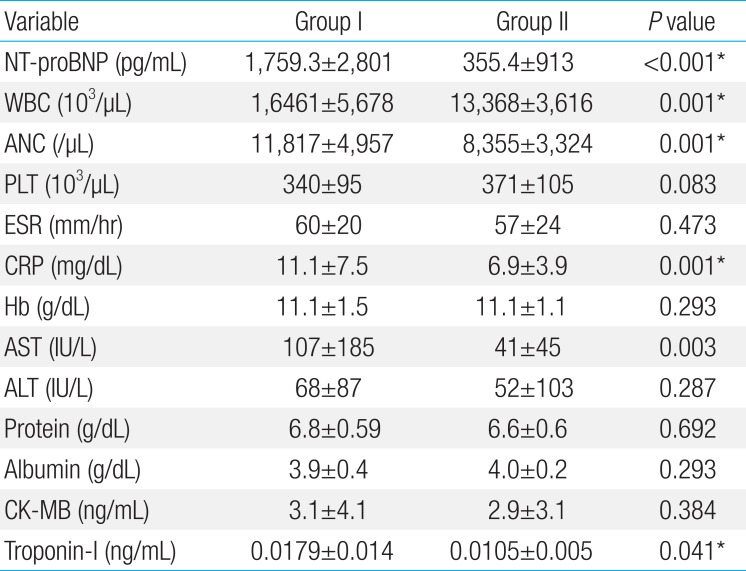
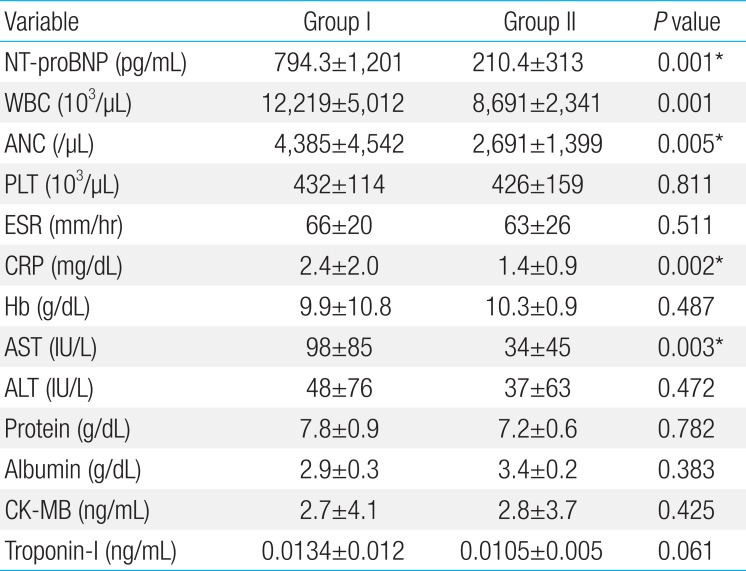
The KD patients were divided into group I (higher plasma NT-proBNP) and II (normal range of plasma NT-proBNP) as previously stated. There were 54 (41%) and 77 patients (59%) with KD in groups I and II, respectively. The gender, body weight, and total hospital days were not different in both group. Fever duration was longer in group I than group II (6.2+2.0 and 4.9+1.5 days, P=0.041) (Table 2). Group I showed significantly higher measurements of WBC, ANC, CRP, AST, and troponin I level (Tables 3, 4). The CAL was developed in 13 (24.0%) in group I and 4 patients (4.5%) in group II. The patient showing IVIG unresponsiveness were 18 (29.4%) and 10 (11.4%) in group I and II, respectively.

The clinical characteristics of KD patients with high level of NT-proBNP (group I) and normal level of NT-proBNP (group II)

Laboratory findings at the time of admission of patients with high level of NT-proBNP (group I) and normal level of NT-proBNP (group II)

Laboratory findings 48–72 hours after completion of intravenous immunoglobulin infusion in patients with high level of NT-proBNP (group I) and normal level of NT-proBNP (group II)
Group I had a significantly higher probability of CAL occurrence (OR, 5.78; 95% CI, 1.771–18.910; P=0.001). IVIG unresponsiveness was three times higher in group I than group II (OR, 3.35; 95% CI, 1.400–9.018; P=0.005) (Table 5).
Discussion
We divided the KD patients into 2 groups according to reference value reported in the literature6) for age adjusted analysis. In group I, fever lasted for a longer time and the serum level of acute phase reactants such as WBC, CRP, ANC were higher than group II. Moreover, AST and troponin-I level were also elevated. In other words, KD patients with serum NT-proBNP level higher than the 95th percentile in the corresponding age group tend to be with severe clinical feature.
Traditionally, plasma NT-proBNP level has been used as an indicator of ventricular dysfunction related to congestive heart failure. Recently, it has emerged as an important biomarker in KD as a tool for diagnosis of incomplete KD23) and to predict the IVIG unresponsiveness or CAL development. There have been several reports that higher plasma NT-proBNP level might also be associated with CAL and myocarditis in KD. Bang et al.4) reported that plasma NT-proBNP level>150 pg/mL in pediatric KD patients is strongly associated with a decrease in myocardial velocity and contractility. Moreover, Kaneko et al.5) stated that plasma NT-proBNP level>1,000 pg/mL is a strong predictor of the presence of CAL in KD patients.
However, the normal range of plasma NT-proBNP levels can vary significantly according to age910). Many studies have reported highly elevated levels of the plasma NT-proBNP level immediately after birth. This is probably related to the physiologic water excretion in the first week following birth and possibly also to the important placental metabolism no longer occurring in this period11). Therefore, single cutoff values without consideration for the age are difficult to apply to individual patients in clinical settings. In particular, dramatic changes in plasma NT-proBNP level occur in infants and young children, which is the KD-prevalent age group. Actually plasma NT-proBNP level of KD patients was inversely correlated with their age in our study (r2=–0.457, P=0.001). Thus, we must consider the age factor in interpreting the concentrations of this peptide.
In our study, group I showed a higher probability of CAL occurrence (OR, 5.78; 95% CI, 1.771–18.910; P=0.001). IVIG unresponsiveness was three times higher in group I than group II (OR, 3.35; 95% CI, 1.400–9.018; P=0.005).
Kim et al.12) reported that higher plasma NT-proBNP levels was identified in the first and second blood tests for IVIG-unresponsive patients, as compared with IVIG-responsive patients. The plasma NT-proBNP level was not statistically different in both IVIG-unresponsive and IVIG-responsive group in our study. We supposed that it was due to small sample size, because P value was quite low (0.051). The risk of IVIG unresponsiveness was three times higher in group I than group II (OR, 3.35, 95% CI, 1.400–9.018, P=0.005). We found that the analysis applying individual upper normal limit of plasma NT-proBNP level on each age categories provided good predictive power for CAL and IVIG unresponsiveness.
In conclusion, age-considering analysis of plasma NT-proBNP level would provide an insight into the disease severity and could be used as a predictive indicator for the the risk of CAL and IVIG unresponsiveness development in KD.
The limitations of the present study are that it was retrospective, and the patient sample size was relatively small.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.