Prognostic factors of neurological outcomes in late-preterm and term infants with perinatal asphyxia
Article information
Abstract
Purpose
This study aimed to identify prognostic factors of neurological outcomes, including developmental delay, cerebral palsy and epilepsy in late-preterm and term infants with perinatal asphyxia.
Methods
All late-preterm and term infants with perinatal asphyxia or hypoxic-ischemic insults who admitted the neonatal intensive care unit of Inje University Sanggye Paik Hospital between 2006 and 2014 and were followed up for at least 2 years were included in this retrospective study. Abnormal neurological outcomes were defined as cerebral palsy, developmental delay and epilepsy.
Results
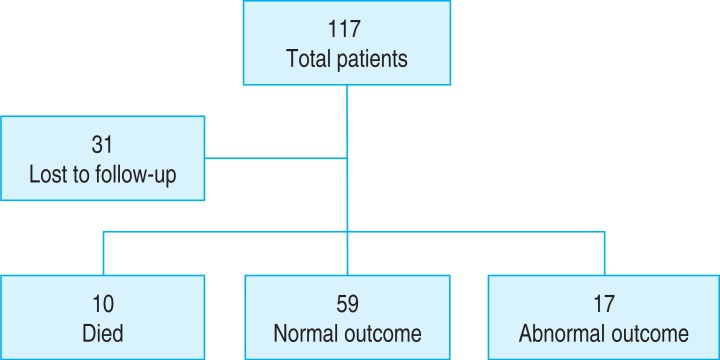
Of the 114 infants with perinatal asphyxia, 31 were lost to follow-up. Of the remaining 83 infants, 10 died, 56 had normal outcomes, and 17 had abnormal outcomes: 14 epilepsy (82.4%), 13 cerebral palsy (76.5%), 16 developmental delay (94.1%). Abnormal outcomes were significantly more frequent in infants with later onset seizure, clinical seizure, poor electroencephalography (EEG) background activity, lower Apgar score at 1 and 5 minutes and abnormal brain imaging (P<0.05). Infants with and without epilepsy showed significant differences in EEG background activity, clinical and electrographic seizures on EEG, Apgar score at 5 minutes and brain imaging findings.
Conclusion
We should apply with long-term video EEG or amplitude integrated EEG in order to detect and management subtle clinical or electrographic seizures in neonates with perinatal asphyxia. Also, long-term, prospective studies with large number of patients are needed to evaluate more exact prognostic factors in neonates with perinatal asphyxia.
Introduction
Perinatal asphyxia, including hypoxic-ischemic encephalopathy (HIE), is a short-term neurological dysfunction with an estimated incidence of 1.5 per 1,000 live births1). Perinatal asphyxia is a frequent cause of neonatal morbidity and mortality, often leading to developmental disabilities, cerebral palsy (CP), and intractable epilepsy2). Factors associated with abnormal outcomes include low Apgar scores at 1 and 5 minutes, moderately severely abnormal electroencephalography (EEG) background findings, and clinical neonatal seizure, as well as the severity and pattern of injuries on magnetic resonance imaging (MRI)/computed tomography (CT)3). Children with normal findings on imaging modalities are more likely to have better outcomes than those with diffuse severe abnormalities. Clinical and electrographic seizures may also be clinical manifestations of neurologic dysfunction in newborn infants, or may cause injury to the brain45). The prognostic significance of many other risk factors is equivocal and may reflect differences in data collection, classification, and analysis. The identification of reliable and remediable prognostic factors about abnormal neurologic outcome would be helpful in the treatment of perinatal asphyxia.
This retrospective study was therefore performed to assess prognostic factors of abnormal neurologic outcomes, especially epilepsy including clinical and electrographic seizures, age at seizure onset, Apgar scores, delivery method, gestational age, EEG findings and abnormal neuroimaging findings in infants with mild to moderate perinatal asphyxia.
Materials and methods
1. Patients and methods
The medical records of late-preterm and term infants (≥35 weeks gestation) with perinatal asphyxia or hypoxic ischemic insults during perinatal period admitted to the neonatal intensive care unit (NICU) of Inje University Sanggye Paik Hospital between 2006 and 2014 and followed up for at least 2 years were retrospectively reviewed. The neonates were identified by searching the electronic medical record for specific diagnosis (i.e., asphyxia, HIE, neonatal seizure) and included using more than 2 of the below criteria2). Patients with cortical malformation, congenital heart disease, or other metabolic or genetic diseases were excluded.
1) Inclusion criteria
-
(1) Signs of fetal distress as indicated by one of following
· Fetal bradycardia (<100 beats/min)
· Blood gas analysis within first hour after birth indicated by a base deficit >16 mmol/L or umbilical artery pH<7.0
Meconium-stained fluid
(2) Apgar score <6 at 5 minute of life.
(3) Patient required positive pressure ventilation before sustained respiration occurs.
(4) Documented changes on EEG consistent with asphyxial injury documented.
(5) Dysfunction in one or more organ system.
(6) Acute changes on neuroimaging consistent with asphyxia.
Factors recorded from medical records included demographic data, age at seizure onset, Apgar scores, EEG findings, and brain imaging findings. In addition, attempts were made to contact the families of these children by telephone to obtain any missing data.
Interictal background EEG activity was graded into 4 categories: normal, mildly abnormal, moderately abnormal, and severely abnormal on the basis of severity of depression, relative amounts of slow activity, and asymmetries within the context of the child's gestational age6). Neurologic outcomes at last follow-up included (1) normal outcomes; (2) CP, defined as a static, nonprogressive motor impairment of early onset characterized by objective changes in muscle tone, muscle strength, posture, abnormal deep tendon reflexes, and motor skills on examination7); (3) developmental delay, defined as less than 85 in mental or psychomotor developmental index of Korean Bayley Scales of Infant and Toddler Development-II (K-BSID-II) or less than 80 of developmental index of Korean infant and child development test (KICDT)89); and (4) epilepsy, defined as recurrent unprovoked seizures that required antiepileptic drugs. The abnormal outcomes were defined when the patient had epilepsy, CP, and delayed development.
The study design was approved by the Institutional Ethical Review Committee at Inje University Sanggye Paik Hospital.
2. Statistical analysis
Continuous variables were compared using t-tests, and dichotomous variables by the chi-square or Fisher exact test, as appropriate. To adjust for possible correlations between putative risk factors and determine independent predictors of outcome, multivariate logistic regression analysis was performed, using variables found to be significant on univariate analysis. Odds ratios and 95% confidence intervals were calculated. All statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL). P-values <0.05 were regarded as statistically significant.
Results
Review of medical records identified 114 late-preterm and term infants diagnosed with perinatal asphyxia during the study period. Of these, 31 were lost to follow-up and excluded (Fig. 1). The remaining 83 infants included 47 boys and 36 girls, of mean±standard deviation (SD) gestational age 38.52±1.91 weeks (range, 35–42 weeks), and the mean±SD birth weight 3,031±521 g (range, 1,860–3,970 g).
The average age of patients was 5.2 years (range, 1–9 years) when this study was conducted and the average age was 4.1 years (range, 1–9 years) when the final outcomes was assessed.
Of these 83 patients, 10 (12.0%) died during NICU admission, 17 (20.5%) had abnormal outcomes and 56 (67.5%) had normal outcomes. Of the 17 with abnormal outcomes, 14 (82.4%) had epilepsy, 13 (76.5%) had CP and 16 (94.1%) had delayed development, including 13 with both CP and delayed development, 10 with both CP and epilepsy, 11 with both epilepsy and delayed development and 10 with CP, epilepsy and delayed development.
Eleven patients were evaluated by K-BSID-II and others were by KICDT for the assessment of the developmental outcomes.
Among the patients with epilepsy, one patient had Lennox-Gastaut Syndrome (LGS), 1 patient had benign childhood epilepsy with centrotemporal spikes, 2 patients had infantile spasm and LGS, and 10 patients had symptomatic focal epilepsy. Of those 10 symptomatic focal epilepsy patients, 2 showed continuous seizure after neonatal seizure.
Of the 83 patients, 43 (52.0%) underwent EEG including long-term video EEG, the indications of EEG were clinically suspected seizures, high risk of acute brain injury, abnormal neurological status (i.e., lethargy or hyperactivity). 81 (97.6%) underwent neuroimaging, including 81 who underwent brain sonography, 62 who underwent brain MRI, and 11 who underwent brain CT. Forty-two patients had clinical seizures (42 of 83, 50.6%).
Comparisons of the groups with normal and abnormal neurological outcomes showed significant differences in age at seizure onset (5.00±5.85 days vs. 2.16±1.42 days, P<0.05), Apgar score at 1 minute (5.86±2.58 vs. 4.26±3.09, P<0.05), at 5 minutes (7.73 ±2.01 vs. 5.92±3.12, P<0.05), occurrence of clinical seizure, brain imaging, and background activity of EEG (Table 1). Moreover, comparisons of groups with and without epilepsy showed significant differences in background activity of EEG, Apgar score at 5 minutes, clinical and electrographic seizures on EEG and brain imaging findings (P<0.05 each) (Table 2).
There were various abnormal brain imaging findings including multiple focal signal changes (17 of 52, 32.7%), high signal intensity or low signal intensity (17 of 52, 32.7%), periventricular leukomalacia (13 of 52, 25%), asymmetric dilation of ventricle (1 of 52, 2%), acute infarction (2 of 52, 3.8%), small gliotic change (1 of 52, 2%), severe brain edema (1 of 52, 2%).
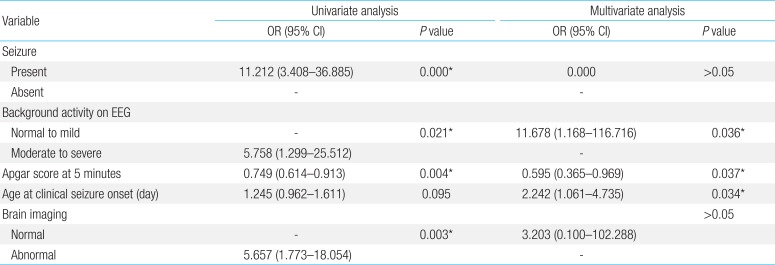
Multiple logistic regression analysis showed that more severe EEG background activity, and later age at seizure onset were significantly predictive of abnormal outcomes in infants with perinatal asphyxia (Table 3). However, we could not found any significant predictors of epilepsy on multivariate logistic regression of infants with perinatal asphyxia because of small number of patients (Table 4).
Discussion
Perinatal asphyxia is the most common cause of acute neurologic morbidity in term infants, as manifested clinically by the symptom complex of neonatal encephalopathy. Moderate or severe neonatal encephalopathy puts the newborn at risk for substantial neurodevelopmental sequelae10). HIE is a short-term neurological dysfunction caused by perinatal asphyxia and is a leading cause of infant mortality and long-term neurologic disability1112). Permanent neurological damage is rare in infants with mild perinatal asphyxia. Moderate encephalopathy, however, is more heterogeneous, with varying rates of disabilities. A recent systematic review of studies evaluating the long-term consequences of HIE found that 14.5% of these infants developed CP2). Predictors of abnormal outcomes included meconium aspiration, low Apgar scores at 1 and 5 minutes, seizure type, abnormal EEG background findings, seizure within 24 hours of birth, and electrographic seizures131415). In our study, 17 patients had abnormal outcomes (17 of 73, 23.3%), including epilepsy, CP, and/or delayed development. Factors significantly higher in infants with abnormal than normal outcomes included clinical seizure, more severe EEG background activity, later age at seizure onset, lower Apgar scores at 1 and 5 minutes, and abnormal brain imaging. Moreover, the risk of developing epilepsy was significantly higher in infants with than without poorer background activity of EEG, lower Apgar score at 5 minutes, clinical and electrographic seizures on EEG and abnormal brain imaging findings.
Seizures are the most common manifestations of neurologic disease during the neonatal period, occurring in about 3.5/1,000 live term births. HIE has been reported to account for more than 60% of early onset neonatal seizures16). Although neurodevelopmental outcomes in term infants with seizures are strongly influenced by the underlying etiology, and diagnostic MRI scans are essential in evaluating underlying brain injury, evidence in both animals and humans has revealed that seizures in themselves may have an adverse impact on immature brains16). Recurrent and prolonged neonatal seizures have been found to act on an epileptogenic substrate, causing further damage, with the latter being responsible for the subsequent clinical manifestations of epilepsy15). We also found that abnormal outcomes were significantly more frequent in infants who did than did not have clinical seizures and that 41.2% (14 of 34) of children who experienced clinical seizures developed epilepsy.
According to previous studies, an earlier seizure with HIE was related with more abnormal outcomes13). In our study, however, abnormal outcomes were significantly more frequent in infants with later than earlier seizure onset. It may be caused by that subtle clinical seizures or electrographic seizures especially at the early stage could be missed because we did not apply continuous EEG monitoring before 24–48 hours of birth in infants with perinatal asphyxia.
Diagnosing neonatal seizures can be quite challenging, primarily for 2 reasons. First, determining whether abnormal movements are the clinical manifestations of electrographic seizures is difficult. Second, 80% to 90% of electrographic seizures do not have any associated clinical correlate and would not be identified without continuous EEG. Thus, neonatal seizure identification and management is often highly dependent on continuous EEG monitoring. Recent guidelines of the American Clinical Neurophysiology Society on Continuous EEG Monitoring in Neonates state that "conventional video-EEG monitoring is the gold standard for neonatal seizure detection and quantification and should be used whenever available for seizure detection and differential diagnosis of abnormal appearing, paroxysmal clinical events1718). Our study showed that 51.8% (43 of 83) underwent EEG, including long-term video EEG, with 12 of these 43 (27.9%) having electrographic seizures and nine of the latter (75.0%) having abnormal outcomes. Of survivals (n=73), 38 patients were done EEG, with 9 of these 38 (23.7%) having electrographic seizures and 6 patients of the latter (66.7%) having epilepsy. Other studies have also revealed that infants treated for clinical and electrographic seizures had a lower incidence of epilepsy, compared with historical controls treated only for clinical seizures1920). These findings suggest that use of long-term EEG in all neonates with perinatal asphyxia would detect more electrographic or subtle clinical seizures. That could make better outcomes and reduce the development of epilepsy in infants with perinatal asphyxia.
In agreement with previous results in term infants31221), we found that moderate to severe background EEG abnormalities were found to be strong predictors of abnormal outcomes and epilepsy. Although amplitude integrated EEG background patterns have been found predictive in asphyxiated infants, EEG is the gold standard method for assessing background activity19). For example, a study of EEG patterns in a mixed cohort of 270 neonates, including 135 full-term babies, found that normal EEG was prognostic of favorable outcomes (normal or minor neurologic sequelae), whereas certain EEG patterns (e.g., inactive or paroxysmal EEG, low voltage with theta rhythm, persistent absence of occipital activity, abnormal anterior activity, fast spikes) were prognostically unfavorable. In addition, Garfinkle and Shevell13) reported that EEG background activity was risk factor for epilepsy in infants with neonatal seizure. EEG is very important tool to predict the prognosis in neonates with perinatal asphyxia.
MRI is widely utilized to assess structural brain abnormalities in high-risk newborns. MRI abnormalities during the neonatal period have been found to be related to later neurodevelopmental outcomes, particularly in infants presenting with HIE1122). A recent study assessed the ability of neonatal MRI to predict neurodisability at 2 years in patients with neonatal seizures, finding that the risks of death or moderate to severe neurodevelopmental impairment were very low in the absence of major cerebral lesions on MRI in patients with HIE. In our study, infants with abnormal brain imaging findings were more likely to have abnormal outcomes than those with normal findings.
Of above these factors, treatable factors such as clinical or electrographic seizures, low Apgar score should be managed. Furthermore, preventive treatments including therapeutic hypothermia and prophylactic medicine are considered to improve neurologic outcomes in neonates with perinatal asphyxia if indicated.
In conclusion, despite the limitations of this study, which include the inclusion of a small number of patients, our findings validate the previously identified prognostic factors after perinatal asphyxia and also found out the risk factors for epilepsy in these patients. Clinical seizures, background activity of EEG, Apgar score at 1 and 5 minutes and abnormal brain imaging findings may be significantly associated with abnormal outcomes. Furthermore, poorer background activity of EEG, lower Apgar score at 5 minutes, clinical and electrographic seizures on EEG, and abnormal brain imaging findings may be related to the risk of developing epilepsy. Therefore, we should apply with long-term video EEG or amplitude integrated EEG in order to detect and management subtle clinical or electrographic seizures in neonates with perinatal asphyxia. Also, long-term, prospective studies with large number of patients are needed to evaluate more exact prognostic factors in neonates with perinatal asphyxia.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.