Enteral nutrition for optimal growth in preterm infants
Article information
Abstract
Early, aggressive nutrition is an important contributing factor of long-term neurodevelopmental outcomes. To ensure optimal growth in premature infants, adequate protein intake and optimal protein/energy ratio should be emphasized rather than the overall energy intake. Minimal enteral nutrition should be initiated as soon as possible in the first days of life, and feeding advancement should be individualized according to the clinical course of the infant. During hospitalization, enteral nutrition with preterm formula and fortified human milk represent the best feeding practices for facilitating growth. After discharge, the enteral nutrition strategy should be individualized according to the infant's weight at discharge. Infants with suboptimal weight for their postconceptional age at discharge should receive supplementation with human milk fortifiers or nutrient-enriched feeding, and the enteral nutrition strategy should be reviewed and modified continuously to achieve the target growth parameters.
Introduction
The current recommendations for nutrition of preterm infants are based on the goal of achieving the growth rate and body composition of a normal fetus at the same postmenstrual age1). However, this goal is often difficult to achieve because of the physiologic limitations associated with prematurity. Postnatal growth restrictions remain a clinical complication of prematurity, especially in extremely low birth weight infants (ELBWI)2). Inadequate nutrition has long been considered a cause of growth retardation. The association between postnatal growth failure due to inadequate nutrition and impaired long-term neurocognitive development is of particular concern. Therefore, a strategy for providing adequate nutrition to ensure optimal growth in premature infants needs to be emphasized.
Principles of enteral nutrition
The American Academy of Pediatrics (AAP) and the European Society of Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition recommended an energy intake of 105–130 kcal/kg/day3) and 110–135 kcal/kg/day4) for preterm infants, respectively. Infants sometimes require increased caloric intake owing to a higher energy demand during an illness. Thus, simply meeting these recommended energy requirements may not be sufficient. Failure to provide adequate protein can result in adverse long-term outcomes in premature infants. Each additional gram of protein intake for ELBWI is associated with additional weight gains of 6.5 g/day and head circumference increase of 0.4 cm/wk5). The National Institute of Child Health and Human Development's Neonatal Research Network evaluated the association between weight gain in the neonatal intensive care unit (NICU) and long-term neurodevelopmental outcomes in ELBWI. Infants in the lowest quartile of in-hospital weight gain (≤12 g/kg/day) were associated with higher odds of cerebral palsy, Bayley scales of Infant Development II mental developmental index <70, and neurodevelopmental impairment at 18–22 months of corrected age, as compared with infants in the highest quartile of inhospital weight gain (≥21 g/kg/day). Similar findings have also been reported for in-hospital head circumference growth6).
These findings suggest that adequate protein intake can improve the growth of ELBWI and that adequate growth in the NICU is associated with a lower risk of long-term neurodevelopmental outcomes. Moreover, an increased protein/energy (P/E) ratio is mandatory to ensure quality growth, presenting as increased lean body mass and limited fat mass deposits. The ESPGHAN recommends protein intakes of 4.0–4.5 and 3.5–4.0 g/kg/day in preterm infants weighing up to 1,000 g and 1,000– 1,800 g, respectively4). With optimal infant growth status, the protein intake can be reduced in anticipation of hospital discharge. However, achieving the recommended protein intake by enteral nutrition remains a difficult task. The protein intake by enteral feeding at 150 mL/kg/day (routine enteral volume) is insufficient for meeting the infant's requirements of 1.8–2.1 g/kg/day of unfortified preterm human milk, 1.5 g/kg/day of unfortified donor human milk, or 3.6 g/kg/day of preterm formula7).
Hence, the question remains as to how much we need to feed ELBWI in order to achieve the recommended daily protein intake.
To provide 4 g/kg/day of protein, we need to feed the infant 290–340 mL/kg/day of unfortified preterm human milk, 210 mL/kg/day of fortified human milk (1 pack/60 mL, Maeil Babywell Human Milk Fortifier, Maeil Co. Ltd., Seoul, Korea), or 170 (16% concentration) to 190 mL/kg/day (14% concentration) of preterm formula (Maeil Babywell Preemie, Maeil Co. Ltd.; Namyang Premie Formula, Namyang Dairy Products Co., Ltd., Seoul, Korea). It is important to note that osmolarity and the renal solute load influence the fluid volumes that are necessary for enteral nutrition. The ESPGHAN reported that 135 and 2004) mL/kg/day should be considered the minimum and maximum fluid volume, respectively. Hence, fortification for human milk-fed infants and adjustment of the formula concentration for preterm formula-fed infants are needed. For routine enteral feeding, preterm formula or fortified human milk that is fed at rates of 150–180 mL/kg/day is sufficient for attaining the recommended nutrient requirements4).
Moreover, as growth in the NICU is associated with long-term neurodevelopmental outcomes, the goal of growth in the NICU may be questioned.
For better neurodevelopmental and growth outcomes in ELBWI, weight gains of >18 g/kg/day and head circumference increase >0.9 cm/wk during the hospitalization period are considered optimal6). If these rates cannot be met, the infant's enteral nutrition should be reviewed and modified to achieve this optimal goal. Focus should be placed on the protein content, P/E ratio, and utilization of nutrient-enriched feeding sources to reduce nutritional deficits.
Practice of enteral nutrition
1. Minimal enteral feeding
Although trophic or minimal enteral feedings involve hypocaloric, low-volume (typically ≤24 mL/kg/day) nutrition to accelerate gastrointestinal, physiological, endocrine, and metabolic maturity, they do not contain sufficient calories to sustain somatic growth8). The most common reason for delays in enteral feeding is the clinicians' concern for the risk of necrotizing enterocolitis (NEC). A recent meta-analysis evaluated the effects of early trophic feeding versus enteral fasting in very low birth weight infants (VLBWI), and found no differences in the feeding tolerance, time to reach full enteral feeding, and incidence of NEC9). Thus, the authors concluded that minimal enteral nutrition was not associated with important beneficial or harmful effects in VLBWI and confirmed the absence of reasons for not starting trophic feeding as soon as possible.
2. Introduction of progressive enteral feedings
The introduction of enteral feeds for VLBWI is often delayed for several days or longer after birth because of concerns that early introduction may not be tolerated and/or may increase the risk of NEC. A systemic review evaluated the effects of early feeding introduction (<4 days) versus delayed feeding introduction (4–7 days) and found no significant differences in terms of the incidence of NEC or all-cause mortality. However, infants with delayed feeding introduction required longer periods before achieving full enteral feedings and delays in hospitaldischarge10).
3. Rate of advancement of enteral feedings
The appropriate rate of enteral feeding advancement has also been the subject of much debate, and an association between rapidly advanced enteral feeding and the risk of NEC has been suggested. However, a recent Cochrane review evaluated the effects of the rate of enteral feeding advancement on the incidence of NEC, all-cause mortality, and other morbidities in VLBWI and found no significant differences between the rates. In the slow advancement group, the infants required a longer period to regain the birth weight and to reach full enteral feeding11). One reasonable approach is to advance enteral feeds by 20–30 mL/kg/day in VLBWI and by 15–25 mL/kg/day in ELBWI7).
Choice of enteral nutrition
1. Human milk
Human milk is the optimal primary nutritional source for preterm infants because it offers strong protection against sepsis and other infections and offers short-term protection against NEC. Regarding the long-term effects, human milk improves neurodevelopmental outcomes, and has a dose-dependent effect until 30 months of corrected age12). Accordingly, the AAP strongly recommends the use of human milk for preterm infants3). Lactation strategies that increase the mother's own milk production should be emphasized in order to enable pumping immediately after delivery1). In the absence of maternal human milk, pasteurized donor human milk is a good option for initiating feeding13). However, despite these benefits, human milk does not completely meet the nutritional needs of preterm infants, owing to insufficient calories and proteins necessary to support the optimal growth and lean body mass accretion. In addition, the concentrations of calcium and phosphorus in human milk are also significantly lower than the required levels7).
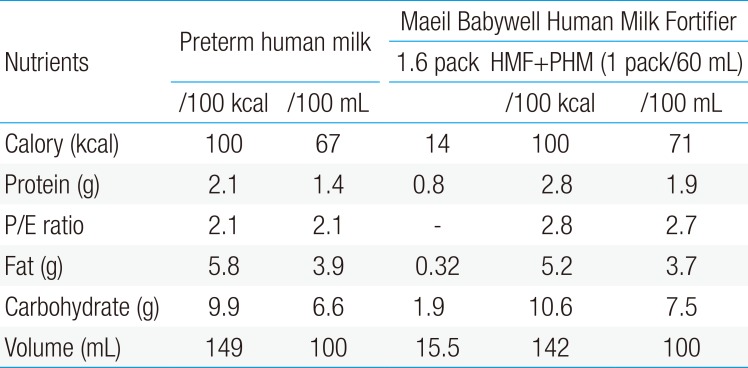
For these reasons, supplementation (fortification) with multinutrient human milk fortifiers (HMFs) is needed in order to meet the nutritional requirements and improve postnatal growth. HMFs are indicated in preterm infants aged less than 31 gestational weeks and/or with a birth weight less than 1,500 g. No definite guidelines regarding the timing for starting HMFs currently exist. However, fortification of human milk does not need to be delayed until full enteral feedings achieved. Generally, fortification commences when the enteral feeding volume reaches 100 mL/kg/day and is gradually increased from half to full strength. Whereas the protein content of human milk declines over the first few weeks from 1.7 g/dL at 7 days to 1.2 g/dL at 42 days, commercial HMFs assume an average protein content of human milk of 1.4–1.6 g/dL7). Therefore, protein content from standard fortification does not reach the amount present in preterm formula, thereby resulting in slower growth compared to preterm formula-fed infants. Consequently, individualized fortification is now believed to be the best solution for inadequate protein intake with standard fortification14). Currently, two methods have been proposed for individualization. Evaluated through periodic determinations of the blood urea nitrogen, targeted and adjustable fortifications are designed based on the analyses of the mother's own milk and de5pend on the metabolic response of each infant, respectively. The use of individualized fortification is recommended to allow adequate protein intake and growth15). The compositions of the commercially available HMFs16) in Korea are shown in Table 1.
2. Preterm formulas
Preterm formula is available for preterm infants who are unable to receive their mother's own milk and donor human milk. In general, these formulas predominantly contain whey protein, glucose polymers, medium-chain triglycerides, calcium, and phosphorus, and they are variably enriched with minerals, vitamins, and trace elements to support intrauterine nutrient accretion rates. The compositions of the commercially available formulas for preterm infants in Korea are shown in Table 2. In practice, a preterm formula of 70–72 kcal/100 mL is initially used. If tolerated by the infants, a higher energy formula consisting of 16% concentrated preterm formula (80–82 kcal/100 mL) may be used to meet the energy demand.
Postdischarge nutrition
At the time of birth, approximately 20% of VLBWI are small for gestational age. After NICU care, however, 79% of these infants experience poor growth and weigh less than the 10th percentile at 36 weeks' postconceptional age (PCA)17). Achieving postdischarge catch-up growth, especially during the vital period between 40 and 48 weeks PCA, is critical for optimal neurodevelopment in premature infants. The rate of weight gain is dependent on the absolute intakes of protein and energy. A high P/E ratio is required to increase lean body mass and protein accretion and to limit fat mass deposition1). Major strategies to reach this goal include continuing preterm formula, human milk alone/partially, full nutrient-fortified human milk, nutrient-enriched postdischarge formula (PDF), and term formula.
1. Preterm formula
The high amount of protein, vitamins, minerals, and electrolytes present in preterm formulas are required in order to meet the increased needs associated with rapid growth, decreased intestinal absorption, and limited fluid tolerance. However, preterm formula is not generally recommended for postdischarge nutrition and is only used until discharge or before switching to PDF.
2. Postdischarge formula
PDF is specially designed for preterm infants after hospital discharge. PDF is less nutrient-dense than preterm formula, but provides energy at 75 kcal/100 mL (vs. 68 kcal/dL for term formula), is protein-enriched (2.1 g vs. 1.4–1.5 g/dL), and contains a variable amount of mineral, vitamins, and trace elements. Although many studies have reported the benefits of PDF, commercial PDF products are currently unavailable in Korea. The use of PDF has been shown to result in more complete catch-up and greater linear growth, weight gain, and bone mineralization than term formula18). Moreover, some studies have reported that after term, PDF cannot change the quantity of growth but rather improves the quality of growth and lowers the fat mass compared to term formula19). Generally, PDF use is recommended until 9-month PCA2). However, a recent meta-analysis concluded that the use of PDF is not supported by the available evidence, because there were no consistent effects on growth at 12–18 months' corrected age20). Further research on the effects of PDF on later growth and neurodevelopmental outcomes are needed.
3. Breast feeding
Most preterm infants are usually discharged by 35- to 36-week PCA, and/or once a weight of 1,800–2,100 g is achieved. However, maximal oral feeding performance occurs by 35- to 37-week PCA. Therefore, preterm infants are unable to manage sufficient volumes to meet their nutritional requirements. For this reason, if the mother plans to breastfeed the infant directly following discharge, the growth rate should be monitored carefully within 48 hours after discharge and reevaluated after 1 week21). For those infants who are discharged while still receiving expressed or donor human milk, fortification is a more practical, continuous option22).
The AAP reported that, by the time of discharge, many VLBWI will have achieved growth at an intrauterine rate but remain below the 10th percentile for their PCA. The AAP further stated that more information is needed on what to feed breastfed preterm infants after discharge, and close growth monitoring is indicated3). On the other hand, the ESPGHAN recommends that infants with an appropriate weight for their PCA at discharge should be breastfed when possible. When formula-fed, such infants should be fed standard formula with long-chain polyunsaturated fatty acids. Human milk-fed infants with subnormal weight for their PCA after discharge should receive supplementation with HMFs to provide adequate nutrients. If formula-fed with suboptimal weight for their PCA, such infants should receive special PDF to decrease the risk of long-term growth failure4). Nevertheless, if the infants in Korea are discharged with a suboptimal weight for PCA, preterm formula is used until a weight of 3.5–4.0 kg is achieved after discharge, enhanced nutrient intake may be achieved by the replacement of one third, half, or two-thirds of daily energy intake with a preterm formula, restricted fortification with HMFs (half strength), or human milk fortified with a preterm formula (80, 90, or 100 kcal/100 mL).
Conclusions
To reach the goals of nutrition for preterm infants, “aggressive” and adequate enteral nutrition are needed. In recent years, focus has shifted from the overall energy intake to the optimal protein intake and P/E ratio. A high overall energy intake may result in excess accretion of adipose tissue, whereas high protein intake may have beneficial effects on the quality of growth and long-term neurodevelopmental outcomes. Minimal enteral nutrition should begin as soon as possible during the 1st day of life, and feeding advancement should be conducted based on the clinical course of each infant. During hospitalization, enteral nutrition with preterm formula and human milk with HMFs are the best feeding practices to improve growth. After discharge, the enteral nutrition strategy should be individualized according to the weight at discharge and should be reviewed and modified continuously to achieve the target growth parameters.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.