Genetics of hereditary nephrotic syndrome: a clinical review
Article information
Abstract
Advances in podocytology and genetic techniques have expanded our understanding of the pathogenesis of hereditary steroid-resistant nephrotic syndrome (SRNS). In the past 20 years, over 45 genetic mutations have been identified in patients with hereditary SRNS. Genetic mutations on structural and functional molecules in podocytes can lead to serious injury in the podocytes themselves and in adjacent structures, causing sclerotic lesions such as focal segmental glomerulosclerosis or diffuse mesangial sclerosis. This paper provides an update on the current knowledge of podocyte genes involved in the development of hereditary nephrotic syndrome and, thereby, reviews genotype-phenotype correlations to propose an approach for appropriate mutational screening based on clinical aspects.
Introduction
Nephrotic syndrome (NS) is a common chronic glomerular disease in children and is characterized by significant proteinuria (>40 mg/m2/hr or a spot urinary protein-to-creatinine ratio of more than 2 mg/mg) and consequent hypoalbuminemia (<3.0 g/dL), which in turn causes edema and hyperlipidemia123). Although NS is associated with many types of renal disease, the most common form (90%) in children is primary (idiopathic) NS, which develops in the absence of clinical features of nephritis or associated primary extrarenal disease. Occasionally, childhood NS can be associated with systemic inflammatory or autoimmune disease or can develop as a result of ischemic insult, infections, drugs, toxins, or inherited renal diseases123).
Most patients (approximately 90%) with idiopathic NS respond to steroid and achieve remission with a good long-term renal prognosis; however, up to 90% of responders will relapse, with approximately half of these patients becoming frequent relapsers or steroid-dependent. The remaining 10%, who continue to have proteinuria after 4 weeks of steroid therapy, are considered to have steroid-resistant nephrotic syndrome (SRNS). The prognosis of patients with SRNS is poor, with 50% developing end-stage renal disease (ESRD) within 15 years12345). To our disappointment, steroid therapy is effective in only about 8%–10% of inherited genetic NS, which subsequently shows multidrug-resistance456). Therefore, SRNS can ensue owing to genetic, idiopathic, or multifactorial pathogenesis. However, with the increase in the identification of specific defects, cases in the idiopathic group are decreasing.
Pathologically, in most cases (~80%, according to the International Study of Kidney Disease in Children [1967–1974] or ISKDC), patients have minimal change NS, which is characterized by steroid responsiveness. In 5%–10% of NS patients (ISKDC), focal segmental glomerulosclerosis (FSGS) is found. Less frequent histologic lesions include mesangial proliferative glomerulonephritis, membranoproliferative glomerulonephritis, and membranous glomerulopathy789). FSGS can be classified as idiopathic, genetic, and secondary caused by injury, medication, or drug abuse based on the underlying causes. Although most histological lesions in genetic NS are unspecific, FSGS is more prevalent than other histologic types10111213). Therefore, because the majority of genetic NS is clinically steroid-resistant and FSGS is more pathologically prevalent, genetic NS is difficult to treat, has poor prognosis, and often progresses to ESRD.
Most children with SRNS present with genetic defects in podocytes. Most cases of genetic NS have monogenic causes and present as an isolated glomerular disease or a syndromic disorder with extrarenal manifestations. Molecular genetic research during the past decade has highlighted the central role of podocytes in the pathogenesis of genetic NS. The ongoing discovery of genetic defects in podocyte-related molecules has significantly added to our understanding of pathophysiology and heterogeneity in genetic NS with respect to pathophysiologic mechanisms, clinical onset, diagnostic assessments, therapeutic approaches, and prognostic judgment13141516). Although genetic testing together with clinical and biochemical investigations in genetic NS will have significant implications on the comprehensive management of NS, genetic diagnosis is far superior to histopathologic classification in predicting responsiveness to immunosuppressants and clinical outcomes13141516). Further research is needed to reveal novel mutations in identified and unknown SRNS podocyte genes. This review focuses on podocyte genes involved in the development of genetic NS and proposes the use of appropriate mutational screenings based on epidemiologic and clinical aspects.
Indications for genetic testing in NS
Genetic testing should be offered to any patient and his/her family afflicted with hereditary NS to help predict the clinical course, responsiveness to immunosuppressive drugs, rate of progression to ESRD, and risk of posttransplant recurrence; thereby, subsequent management of SRNS can be tailored accordingly13).
Although no consensus exists, appropriate genetic testing for SRNS-related mutations can be determined on the basis of current evidence in scenarios with (1) a positive family history of SRNS, (2) congenital or infantile onset (<1 year) of SRNS, (3) SRNS with onset before 25 years of age, (4) lack of response to immunosuppressive drugs, (5) histological findings of idiopathic FSGS or diffuse mesangial sclerosis (DMS) on renal biopsy, (6) presence of extrarenal manifestations (syndromes), (7) reduced renal function or renal failure, and (8) certain ethnic groups1317).
Ongoing genotype-phenotype studies of NS patients with several genes and genetic variants have identified correlations with clear clinical significance. When supplemented with hereditary, clinical, laboratory, and/or histologic data obtained, genetic testing results would allow the clinician to refine their diagnostic and therapeutic understanding of an individual case of NS. This could guide a more accurate prognosis or family counseling for a patient or optimize the clinical decision making process in terms of prenatal diagnosis, genotype-phenotype correlations, choice of pharmacotherapy and dosage, or appropriate frequency or type of clinical monitoring, including risk assessment of recurrence after kidney transplantation1819).
Genetic methods in hereditary NS
Historically, genetic research in NS has studied families with SRNS in order to discover rare mutations in single podocyte genes that lead to this disease131420). The methodology of genetic testing is changing rapidly. Traditionally, genetic testing in diagnostic laboratories involved the Sanger sequencing of known disease-related genes. However, recent technical advances in high-throughput sequencing allow a high diagnostic yield and a continuous reduction in cost172122).
Sanger sequencing is a straightforward and highly sensitive tool to test one or few genes per patient; however, in genetically heterogeneous disorders with multiple causal genes, Sanger sequencing of all known and suspected genes is time-consuming and not cost-effective23). Thereafter, Sanger sequencing for the detection of a single causational gene of SRNS has been replaced by high-throughput sequencing techniques like next-generation sequencing (NGS); however, Sanger sequencing still plays a very important role for the confirmation of genetic variants identified using high-throughput sequencing techniques.
Massive parallel NGS encompasses several high-throughput sequencing approaches and can provide the selective sequencing of a small number of genes, the coding portion of the genome (the exome), or the entire genome. Using NGS technologies, most monogenic SRNS genes can now be determined within days by utilizing a single blood test and at competitive prices compared with traditional Sanger methods. NGS can be used to identify new genes in SRNS or to simultaneously study the mutational spectrum of many genes in SRNS13212224). Gene panel analysis performed using NGS currently has higher traction in clinical use compared to whole-exome sequencing (WES) or whole-genome sequencing (WGS), because of its cost-effectiveness. Gene panels are optimized for target sequence coverage and consequently have higher read depth and accuracy compared to the WES or WGS output; however, the number of genes is limited to ~30. Genome-wide association studies (GWAS) typically focus on associations between single-nucleotide polymorphisms and traits such as major diseases. GWAS of cohorts of unrelated, affected subjects with SRNS can uncover disease-associated loci20). These and other approaches including enrichment and DNA pooling can complement rare variant discovery17202122).
Glomerular filtration structure
NS is a consequence of primary increase in the permselectivity barrier of the glomerular capillary wall, leading to passage of proteins through the defective filtration barrier123). Therefore, elucidating the glomerular filtration structure is mandatory for understanding the development of NS.
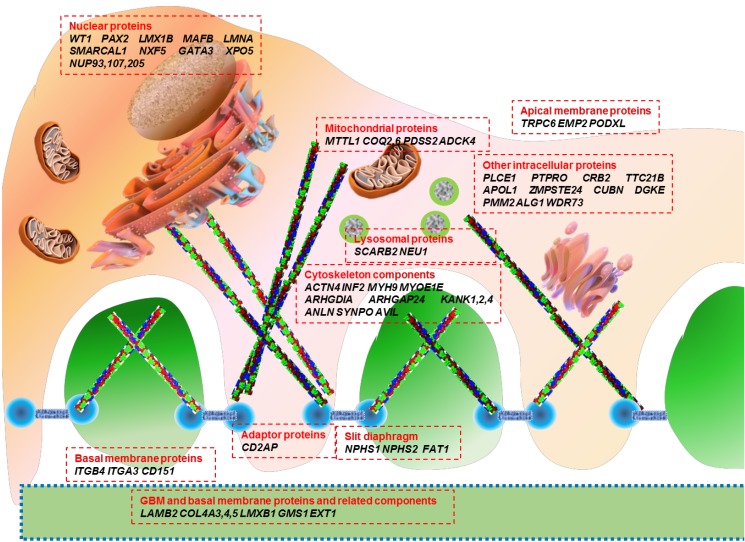
The glomerulus is a tuft of capillary loops supported by the mesangium, and is enclosed in a pouch-like extension of the renal tubule, known as the Bowman capsule. The glomerulus consists of 4 types of resident cells (mesangial, glomerular endothelial, visceral epithelial [known as the podocyte], and parietal epithelial), and 3 major types of matrices (mesangial, glomerular basement membrane [GBM], and Bowman basement membrane). The renal filtering apparatus of the glomerular capillary tuft consists of three major components: the inner fenestrated endothelial cell layer, the GBM, and the outer podocyte layer252627).
The highly specialized and terminally differentiated podocytes branch off cytoplasmic processes to cover the outside of the glomerular capillary, with primary major, secondary minor, and tertiary processes—the so-called ‘foot processes’ (FPs)252627). The podocyte cell body contains a prominent nucleus, a well-developed Golgi system, abundant rough and smooth endoplasmic reticulum, prominent lysosomes, and abundant mitochondria. The high density of organelles in the cell body indicates high levels of anabolic as well as catabolic activities19). In contrast to the cell body, the cytoplasmic processes contain few organelles. In the cell body and primary processes, microtubules and intermediate filaments such as vimentin and desmin dominate; whereas, microfilaments, in addition to a thin cortex of actin filaments beneath the cell membrane, densely accumulate and form loop-shaped bundles in the FPs272829).
The FPs of podocytes regularly interdigitate with those from neighboring podocytes, leaving between them meandering filtration slits about 25–40 nm in width, which are bridged by the slit diaphragm (SD). The filtration slits between SDs are critical for the filtering process through the podocyte FPs and retention of protein in the blood stream262730). The SD comprises nephrin and other related structural proteins that are linked intimately to the actin-based cytoskeleton through adaptor proteins such as CD2-associated protein (CD2AP), zonula occludens-1, β-catenin, Nck, and p130Cas, located at the intracellular SD insertion area near lipid rafts262728293031). Signaling pathways from these SD molecules through adaptor proteins regulate podocyte structure, cell adhesion, cell survival/apoptosis, differentiation, and cellular homeostasis262831).
Recently, the structures of the SD and podocyte molecules have become the mainstay of many studies, and several novel proteins have been found to be important for their function and the development of genetic and nongenetic NS.
Podocyte genes associated with hereditary NS
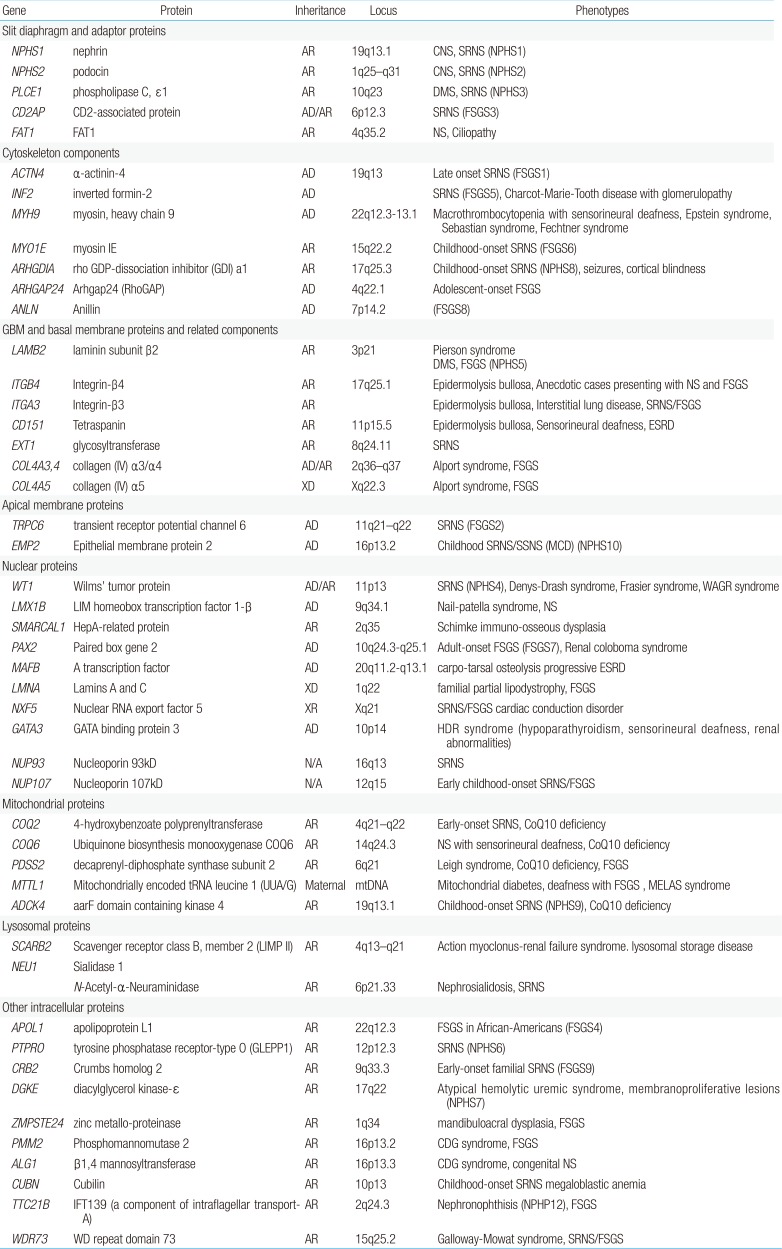
Currently, over 45 recessive or dominant genes have been associated with SRNS/hereditary NS in humans; the known podocyte genes explain not more than 20%–30% of hereditary NS. However, they explain 57%–100% of familial and infant-onset NS, as compared with 10%–20% of sporadic cases1524).
Genetic mutations affect proteins that are expressed at a variety of locations within the podocyte, including the cell membrane, nucleus, cytoskeleton, mitochondria, lysosomes, and intracellular cytoplasm (Fig. 1). The podocyte proteins uncovered so far belong to the following categories: (1) located at the SD and connected adaptor proteins, (2) involved in regulation of actin dynamics, which are essential for the maintenance of podocyte structure and function, (3) adhesive proteins at the basal membrane and GBM components, (4) apical membrane proteins related to cell polarity, (5) nuclear transcription factors, (6) proteins belonging to intracellular organelles such as mitochondria and lysosomes, which are central players in podocyte metabolism, and (7) other intracellular proteins (Table 1)3233343536).
Genotype-phenotype correlations in monogenic SRNS
Advances in podocytology and genetic techniques have expanded our understanding of the pathogenesis of hereditary SRNS, and have accelerated elucidation of the mechanism of normal and pathologic glomerular filtration through podocytes. Further, knowledge on genetic and nongenetic podocytopathies add to the genetic and phenotypic data. Genotype-phenotype correlations in hereditary NS have been explained well, and will be reviewed here.
1. Recessive versus dominant inheritance
There is significant phenotypic variability associated with defects in NS-related genes. Recessive mutations in NPHS1, NPHS2, LAMB2, and PLCE1 cause severe clinical features of early-onset NS and progress to ESRD, either during infancy or throughout childhood. Recessive mutations in CD2AP and MYO1E cause severe childhood-onset NS and progress to ESRD later. Hereditary, autosomal-dominant NS is rare, occurring mostly in juvenile and adult familial cases. Dominant mutations in ACTN4, TRPC6, and INF2 are associated with late-onset proteinuria and progress to ESRD during the third and fourth decades of life13141737). Inheritance patterns for recently reported genetic mutants responsible for hereditary NS are listed in Table 1.
For recessive mutations, family history is unlikely, because parents of individuals harboring such mutations will be healthy heterozygous carriers, and no one in the ancestry will have had disease (because if there is any inherited mutation, it will be heterozygous). For dominant mutations, one of the parents of the affected individual will most likely be affected, and the disease may have been handed down through multiple generations (except for situations of de novo mutations or incomplete penetrance, which can occur in case of autosomal-dominant genes). Thereby, the detection of dominant mutations has important clinical implications in situations of living-related donor selection in kidney transplantation17).
2. Age-at-onset and specific genes
NS that presents at birth or within the first 3 months of life is termed congenital nephrotic syndrome (CNS), while the term infantile nephrotic syndrome (INS) is used for NS presenting between 3 and 12 months of age. Later-onset NS includes early/late childhood (1–5 years/thereafter) and adult-onset NS.
CNS is usually caused by a defect in a major podocyte SD gene, nephrin (NPHS1), associated with Finnish type nephropathy. In non-Finnish populations, CNS is actually a clinically and genetically heterogeneous group of disorders caused by mutations in WT1, PLCE1, LAMB2, or NPHS2. Therefore, early-onset NS (CNS and INS) is usually caused by mutations in NPHS1, or less commonly by mutations in WT1, PLCE1, LAMB2, NPHS2I, or LMX1B1432333437383940). Patients with CNS mostly presented steroid-resistant proteinuria in the first few days of life, and rapidly progressed to ESRD by the age of 2–8 years; NPHS1 mutations were detected in more than half of all cases. However, some NPHS1 mutations are associated with ESRD occurring after the age of 20 years3841424344). As an example, a case series from New Zealand reported that Maori children with CNS have indolent clinical course and prolonged renal survival44). Mutations in TRPC6, CD2AP, or MYO1E cause severe childhood-onset NS.
Most cases of adult-onset familial FSGS are inherited as autosomal-dominant disease. The most common causative gene is INF2 (up to 17%); other mutations include those in TRPC6 (up to 12%), ACTN4 (3.5%), PAX2, and SCARB215). However, penetrance is often incomplete, with variable expression. Many adult patients with familial FSGS present with nonnephrotic proteinuria.
3. Renal pathology
Podocytopathies include 4 histologic patterns of glomerular injury: (1) minimal change nephropathy (MCN), if the number of podocytes per glomerulus is unchanged; (2) FSGS, if there is podocytopenia with segmental glomerulosclerosis; (3) DMS, where low proliferative index has been described; or (4) collapsing glomerulopathy, if there is marked podocyte proliferation with collapsed capillaries4546). For specific genes and specific mutations within the same gene of podocytes, there can be a correlation between genotypes and renal histologic patterns1737).
In infants (CNS and INS) DMS was a frequent histologic finding, whereas FSGS was observed in up to 90% of individuals with proteinuria onset at 7 to 25 years47). Individuals with CNS and the renal histology of DMS usually have mutations in the following genes: PLCE1, LAMB2, WT1, NPHS1, NPHS2, or GMS1; however, MCN and FSGS are rarely seen in CNS1737384647). Data from human genetics and from mouse models of SRNS/FSGS show that the renal histologic patterns of DMS and FSGS lie at different ends of the spectrum of a shared pathogenesis. This means that “severe” recessive mutations (protein-truncating mutations) in NPHS2 cause a fetal-onset renal “developmental” phenotype of immature glomeruli (i.e., DMS), whereas “mild” mutations (missense mutations) in the same gene, NPHS2, cause the late feature of renal “degenerative” phenotype, FSGS1748). Irregular microcystic dilation of the proximal tubules is a characteristic histologic feature in patients with mutations in NPHS1; and this condition may be useful for differentiating patients with NPHS1 mutations from those with NPHS2 mutations; however, this is not observed in all cases38).
Familial FSGS may have clinical characteristics that are similar to sporadic FSGS, but the prognosis is different. Patients with familial FSGS tend to be unresponsive to steroid therapy. Thus, when abundant proteinuria is present, the prognosis of familial FSGS is poor1549).
4. Syndromic versus nonsyndromic NS
Mutations in specific SRNS genes may cause distinct clinical phenotypes in a gene-specific and/or allele-specific manner1748). Genes implicated in nonsyndromic podocytopathies are NPHS1, NPHS2, CD2AP, PLCE1, ACTN4, TRPC6, INF2, and others14151750). In syndromic forms of FSGS, the extrarenal manifestations are most prominent and often diagnostic. Syndromic forms of SRNS, which are far less frequent, may be a result of mutations in genes encoding transcriptional factors (WT1, LMX1B), GBM components (LAMB2, ITGB4), lysosomal (SCARB2) and mitochondrial (COQ2, PDSS2, MTTL1) proteins, a DNA-nucleosome restructuring mediator (SMARCAL1) or cytoskeletal proteins, including inverted formin 2 (INF2) and nonmuscle myosin IIA (MYH9)14151751).
Mutations in the WT1 gene, encoding the Wilms' tumor 1 protein, which typically lead to Denys-Drash syndrome or Frasier syndrome, can also cause isolated SRNS52). In addition, mutations in LAMB2, encoding laminin-β2, are usually implicated in Pierson syndrome associated with neuronal or retinal involvement5354). Individuals with LMX1B mutations usually present with Nail-Patella syndrome. However, specific LMX1B mutations have been found in individuals with isolated SRNS55). INF2 mutations can either lead to isolated NS or be present in individuals with Charcot-Marie-Tooth disease56). MYH9, a podocyte-expressed gene encoding non-muscle myosin IIA, has been identified as the disease-causing gene in the rare giant-platelet disorders, including May-Hegglin anomaly, Epstein-Fechtner, or Sebastian syndrome57). Genetic causes of most syndromic NS are summarized in Table 2.
Conclusions
According to genotype-phenotype correlations in hereditary NS, an appropriate genetic approach for patients with hereditary NS could be determined based on inheritance, age at onset, renal histology, and presence of extrarenal malformations.
Acknowledgments
This review was supported by the Clinical Research Fund of Chungbuk National University Hospital and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A4A03006207).
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.