Posttransplantation lymphoproliferative disorder after pediatric solid organ transplantation: experiences of 20 years in a single center
Article information
Abstract
Purpose
To evaluate the clinical spectrum of posttransplantation lymphoproliferative disorder (PTLD) after solid organ transplantation (SOT) in children.
Methods
We retrospectively reviewed the medical records of 18 patients with PTLD who underwent liver (LT) or kidney transplantation (KT) between January 1995 and December 2014 in Seoul National University Children's Hospital.
Results
Eighteen patients (3.9% of pediatric SOTs; LT:KT, 11:7; male to female, 9:9) were diagnosed as having PTLD over the last 2 decades (4.8% for LT and 2.9% for KT). PTLD usually presented with fever or gastrointestinal symptoms in a median period of 7 months after SOT. Eight cases had malignant lesions, and all the patients except one had evidence of Epstein-Barr virus (EBV) involvement, assessed by using in situ hybridization of tumor tissue or EBV viral load quantitation of blood. Remission was achieved in all patients with reduction of immunosuppression and/or rituximab therapy or chemotherapy, although 1 patient had allograft kidney loss and another died from complications of chemotherapy. The first case of PTLD was encountered after the introduction of tacrolimus for pediatric SOT in 2003. The recent increase in PTLD incidence in KT coincided with modification of clinical practice since 2012 to increase the tacrolimus trough level.
Conclusion
While the outcome was favorable in that all patients achieved complete remission, some patients still had allograft loss or mortality. To prevent PTLD and improve its outcome, monitoring for EBV infection is essential, which would lead to appropriate modification of immunosuppression and enhanced surveillance for PTLD.
Introduction
Posttransplant lymphoproliferative disorder (PTLD) is a well-known, potentially fatal complication of solid organ transplantation (SOT) with high mortality (30%–60%)1). The incidence of PTLD in SOT recipients varies from 1% to 13% depending on the type of allograft, ages of recipients and the length of follow-up23). The majority of PTLD cases are associated with Epstein-Barr virus (EBV) infection, either primary infection or reactivation due to immunosuppression4). Risk factors of PTLD include EBV-naïve recipients with EBV-positive donors, younger age at SOT, more aggressive immunosuppression, and within the first year of SOT5).
In pediatric allograft transplantation, PTLD is relatively common because the recipients are often EBV seronegative at the time of transplantation5). As Korean experience, Heo et al.6) had reported 5 cases of PTLD among 41 pediatric liver allograft recipients (12.2%) in 2004. Another study of 43 Korean PTLD cases reported 12 pediatric cases, but the incidence among pediatric SOT recipients was not described7). Recently, our center has experienced several consecutive cases of pediatric PTLD, which lead us to review the clinical spectrum of PTLD in pediatric SOT recipients. In this study, we present our experience of pediatric PTLD of 20 years.
Materials and methods
We retrospectively reviewed the medical records of PTLD cases among pediatric SOT recipients of liver (LT) or kidney transplantation (KT) that had been performed from January 1995 to December 2014 at Seoul National University Children's Hospital. The study was approved by the Institutional Review Boards of our center (IRB#H-1312-068-541). PTLD was clinically suspected when SOT recipients presented persistent lymphadenopathy or tumorous lesions, which were confirmed pathologically and classiied following the classification of the World Health Organization system8). We categorized the patients into the malignant group and the benign group according to the pathology. Early PTLD was defined as PTLD occurring within the first year of SOT5). EBV association was assessed in tissue specimens by in situ hybridization of Epstein-Barr virus encoded RNA. EBV viral loads in the patients were measured by real-time polymerase chain reaction of EBV DNA in the peripheral blood of the patients.
Characteristics of the patients were reviewed as follows: clinical features at the time of transplantation (underlying disease, transplanted organ, age at transplantation, EBV serologic status of the donor and the recipient at transplantation, Human Leukocyte Antigen [HLA] typing); posttransplantation history (rejection episode, immunosuppressive treatment, cytomegalovirus [CMV] reactivation status); and characteristics of PTLD (clinical presentation, histopathologic diagnosis, expression of CD20 on tumor cells, detection of EBV in tumor cells, stage, treatment, outcome, graft survival, overall survival [OS]). OS is defined as the duration from diagnosis of PTLD to the last follow-up or death. Clinical staging was performed retrospectively according to the Ann Arbor staging system for lymphoma9).
Differences between PTLD patient subsets were assessed by chi-square test for categorized variables and Welch-Aspin test for continuous variables. A P value of less than 0.05 was considered statistically significant. The statistical analysis was performed using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA).
Results
1. Characteristics of PTLD patients (Table 1)
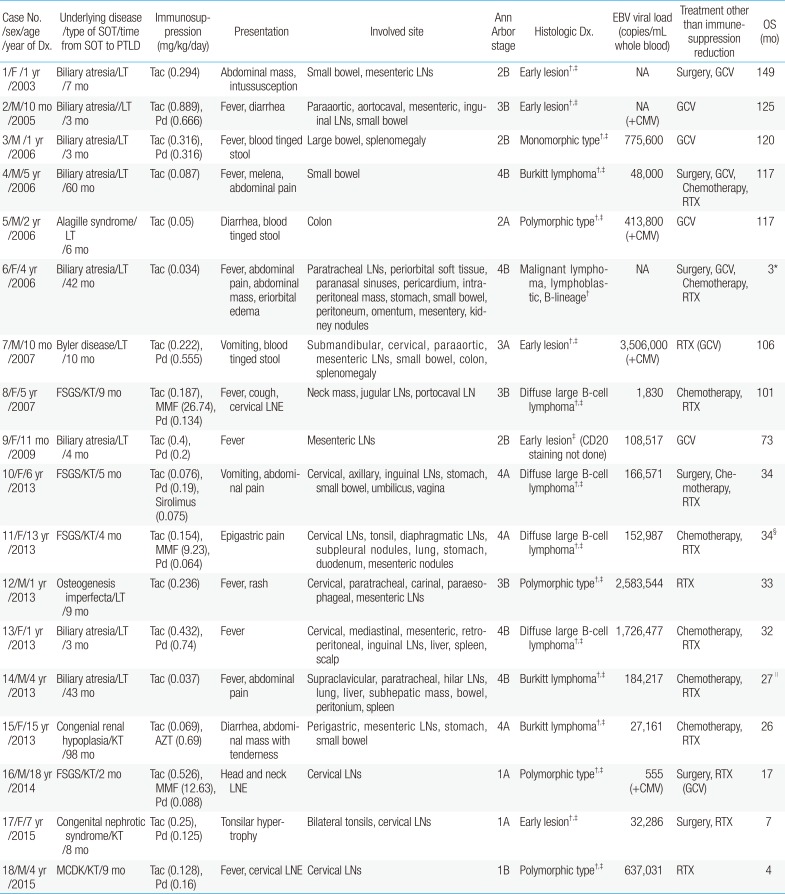
Over the last 2 decades (January 1995 to December 2014), 18 patients (LT:KT, 11:7) were diagnosed with PTLD, rendering the incidence of PTLD 4.8% for pediatric LT (of total 230 recipients) and 2.9% for pediatric KT (of 234 cases). For these PTLD cases, median age at transplantation was 11 months (range, 3 months to 18 years) and PTLD was diagnosed at median 7 months (range, 2 to 98 months) after SOT, mostly during the first year (n=14, 77.7%), at their median age of 51 months (range, 10 months to 18 years). KT patients with PTLD were older at SOT (median age, 76 months; range 44 months to 13 years) than LT recipients (median age, 7 months; range, 3 to 18 months) on their diagnosis of PTLD.
All patients with PTLD were taking tacrolimus targeting trough levels of 10–15 mg/mL in immediate postoperation periods, then 6–8 mg/mL (before 2012) or 8–10 mg/mL (since 2012) for KT and 5–8 ng/mL for LT. Basiliximab was administered in eight patients as induction immunosuppression, and 6 of LT patients with PTLD were treated for acute rejection before diagnosis of PTLD. EBV serology of the recipients at the time of SOT was antibody negative in 7, positive in 5, and unknown in 6. HLA types of the patients were predominantly HLA-A2, A33, B44, B58, DR13, and DR15. None of the patients had malignancy before SOT.
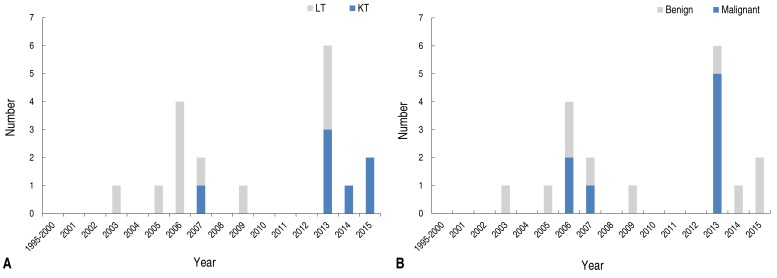
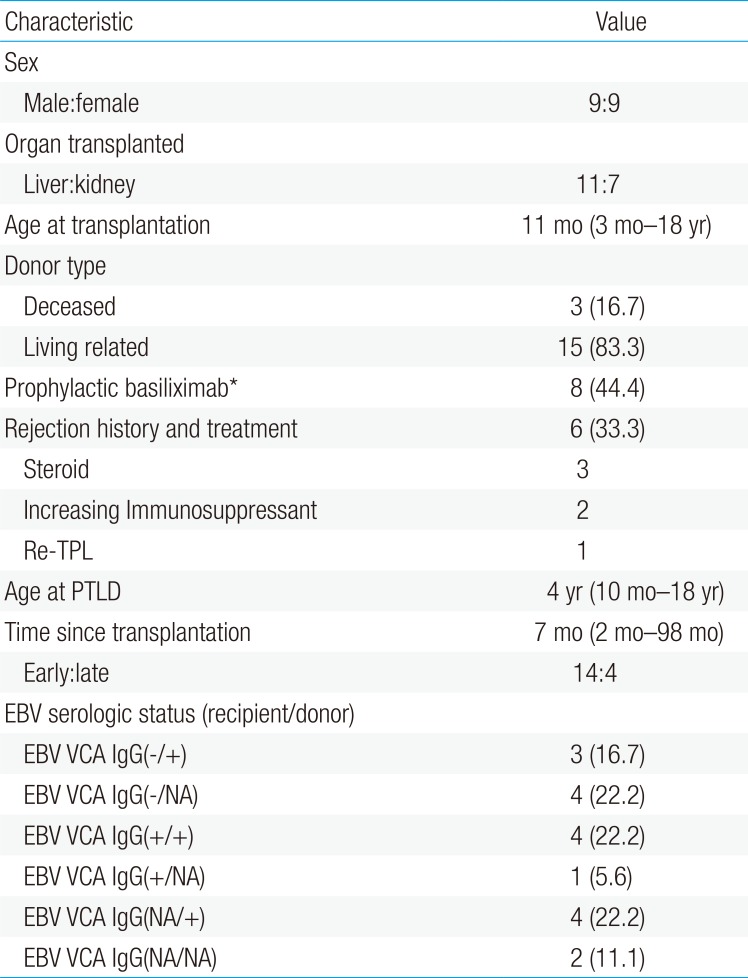
Since we recently experienced several consecutive cases of PTLD we compared recent cases with those before 2012 (Table 2). While KT cases were more common after 2012 along with different age at SOT and PTLD (Fig. 1A), there were no statistically significant differences in time interval between SOT to PTLD, immunosuppression, EBV viral load, or pathologic malignancy (Fig. 1B).
2. Presentation of PTLD (Table 3)
Initial symptoms of PTLD were mostly fever (n=10, 55.6%) or gastrointestinal problems, such as abdominal pain, vomiting, diarrhea, and blood tinged stool (n=11, 61.1%). Fifteen patients had lymph node enlargement (83.3%) and 3 patients had mass or nodular lesions (16.7%), mostly in the intraperitoneal cavity. The majority of the patients had gastrointestinal organ involvement, including small bowel (n=9, 50%) and intraperitoneal lymph nodes (n=8, 44.4%).
Pathologic diagnosis of PTLD revealed 5 cases of early lesion (27.8%), 4 cases of polymorphic type (22.2%), 1 case of monomorphic type (5.6%), 4 cases of diffuse large B-cell lymphoma (22.2%), 3 cases of Burkitt lymphoma (16.7%), and 1 case of malignant lymphoblastic lymphoma (5.6%). CD20 expression of tumor was positive in all patients tested (n=17), suggesting B-cell proliferation. Seventeen patients showed EBV positive-tumors (94.4%). At the time of PTLD diagnosis, EBV viral loads were 555–3,506,000 copies/mL whole blood (median, 166,571 copies/mL) when measured (n=15). Four patients showed evidence of CMV coinfection by CMV IgM seroprevalence, CMV antigenemia or CMV viral load, reactivation in two and de novo infection in others.
When compared to benign PTLD patients (median, 6.5 months after SOT), those with malignant PTLD seemed to be diagnosed with PTLD later (median, 9 months after SOT), although there was no statistically significant difference (Table 4), and female patients seemed to be common in the malignant group (P=0.058). There was no statistically significant difference in B symptoms, involved organs, or EBV titers at diagnosis of PTLD.
3. Treatment and outcome
Upon recognition of PTLD, maintenance immunosuppression was reduced in all patients, similar to what was reported in the literature1011), and additional treatments were applied as appropriate (Table 3, Fig. 2). Surgery was considered as the primary treatment for localized lesion and primary tumors were removed by surgery in one third of the patients (n=6, 33.3%). Rituximab (RTX) was administered in every case since 2013 and one malignant case in 2006 (72.2%). Before RTX was established as the first line treatment for PTLD, ganciclovir (GCV) had been tried as an initial treatment of PTLD (n=6). GCV was also used along with RTX when there was CMV coinfection (patient numbers 7 and 16). Chemotherapy was administered for malignant PTLD (n=8). The first line chemotherapy in our institute was CCG 106B protocol12) (prednisolone, vincristine, daunomycin, cyclophosphamide, and intrathecal chemotherapy of cytarabine, hydrocortisone, and methotrexate) along with RTX.
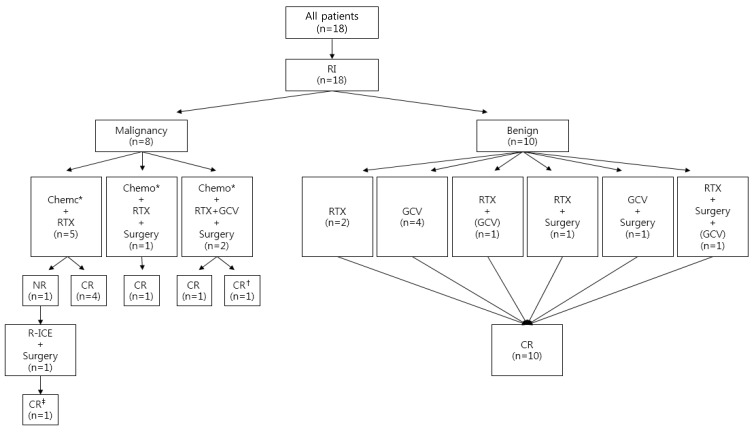
Treatment and response. RI, reduction of immunosuppression; Chemo, chemotherapy; RTX, rituximab; GCV, ganciclovir; NR, no response; CR, complete remission; R-ICE, rituximab, ifosfamide, carboplatin, etoposide. *CCG 106B protocol. (GCV) GCV was used for CMV coinfection. †Expired from complication of chemotherapy (sepsis) after remission of posttransplantation lymphoproliferative disorder (PTLD). ‡Graft loss during chemotherapy after remission of PTLD.
All patients achieved complete remission of PTLD (Fig. 2). Four patients achieved remission with GCV monotherapy, and 2 patients (patient numbers 12 and 18) achieved remission with RTX monotherapy. Median OS was 35 months (range, 3 months to 12 years). However, patient number 11 lost her allograft kidney during chemotherapy, and patient number 6 expired from complication of chemotherapy (sepsis) with functioning graft after achieving remission of malignant lymphoma. At last follow-up, all the surviving patients showed no evidence of PTLD, and their EBV titers were median 3,145 copies/mL (range, 0–43,101 copies/mL).
DISCUSSION
In the early 2000s, our center experienced its first PTLD cases. Similar to the observations of others5) this coincided with the introduction of tacrolimus, which replaced cyclosporine as the main immunosuppressant at our institute in June 2001. After starting regular monitoring of EBV viral load in 2007 and adjusting immunosuppressant accordingly, PTLD cases at our center decreased. However in 2013, recent consecutive cases of PTLD emerged once again. The majority of recent cases were KT recipients and the rate of malignant lymphoma seemed high, while statistically not significant. Changes in the clinical practice for KT recipients before the recent emergence of PTLD patients were not evident, except that the target trough level of tacrolimus was increased from 6–8 to 8–10 ng/mL since 2012. Otherwise characteristics of PTLD patients were similar between those before 2012 and after 2012, except their age at SOT; Generally LT patients are younger than KT patients at SOT, since their common cause of organ failure is biliary atresia in Korea13), and PTLD incidence is higher in LT patients along the line that younger age at SOT is a known risk factor of PTLD. While our number of patients was too small to do statistically appropriate analysis, it seems that with higher target trough level of tacrolimus, the main immunosuppressive agent for SOT, rendered KT patients after 2012 prone to PTLD, as much as younger age of recipient does.
Nevertheless, the clinical spectrum of our cases of PTLD were similar to what is known; the median age at the time of PTLD (4 years) and interval between PTLD diagnosis and transplantation (7 months) of our pediatric PTLD cohort are similar to those reported by Opelz and Döhler5). We achieved complete remission in every case with a low rate of graft loss (5.6%), which is much higher than reported in the early 2000's1415) but similar to those of a recent report16). In order to assess risk factors of PTLD among pediatric SOT recipients, comparison between patients with PTLD and without PTLD should be done, which is beyond the scope of this report and it is the limitation of this report; Among the known risk factors14), frequency of CMV infection in our cases was 22%, not different from known frequency of CMV in pediatric SOT recipients of one forth17). HLA types of recipients A2, A11, A26, B5, B8, B18, B21, B38 and B40 had been reported to be of risk of PTLD18192021), and in our cases HLA-A2 was common; however in Korean population HLA A2 is common15), therefore significance thereof is yet elusive.
What called our attention during our review of pediatric PTLD cases again was that patients who were diagnosed at their early disease status suffered much less than those who found to have PTLD at later stage. While all the patients achieved remission eventually, those malignant cases had to experience long periods of chemotherapy and concomitant complications, including neutropenic fever, graft loss, and death due to sepsis. On the other hand, RTX with reduction of immunosuppression was enough to induce complete remission if the lesion was localized and benign, as experienced by patient numbers 12 and 18. These 2 patients were under surveillance because there was a sudden increase of EBV viral load, therefore more careful physical examination could detect well-defined benign lesion rather early, and they achieved remission without any severe complication. Therefore, we emphasize that early diagnosis and early intervention with appropriate management is necessary for good outcome of PTLD as reported in many studies162223).
For early detection and furthermore prevention of PTLD, monitoring EBV viral load is considered as one of the crucial factors, even though the specificity and positive predictive value of EBV titer in predicting PTLD are still controversial24252627). As an example, right after that regular EBV viral load monitoring was adopted as standard clinical practice, our center encountered only one case of pediatric PTLD during 2008–2012; Upon recognition of high titer of EBV of >10,000 copies/mL immunosuppression was reduced when possible, and active surveillance of PTLD was adopted. While active monitoring of EBV viral load might not enough to overcome the hazardous effect of more aggressive immunosuppression as suspected as predisposing factor for our recent PTLD cases, we need to monitor EBV infection meticulously, since this is the only tool available to prevent PTLD.
In conclusion, during 20 years of SOT, our center experienced 18 cases of pediatric PTLD (3.9% of pediatric SOT). Most of the patients presented with abdominal symptom, and 44% were malignant. Higher incidence of PTLD coincided with the introduction of Tac and probably also with a higher target trough level of Tac. The outcomes of our PTLD patients were favorable with remission achievement in all patients and a low rate of graft loss and mortality. While previously considered as a fatal complication of SOT, the outcome of PTLD has much improved these days, and active monitoring of EBV infection might reduce this complication of pediatric SOT even further.
Acknowledgment
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C0014).
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.