Neonatal indirect hyperbilirubinemia and glucose-6-phosphate dehydrogenase deficiency
Article information
Abstract
Purpose
This study aimed to determine the prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency among infants with neonatal indirect hyperbilirubinemia (NIH); compare G6PD-deficient and G6PD-normal patients regarding hyperbilirubinemia and need for exchange transfusions (ET); and assess risk factors for ET and kernicterus.
Methods
This is a case-control retrospective study. Medical records of NIH patients admitted to the Pediatric Department, Salmaniya Medical Complex, Bahrain, between January 2007 and June 2010 were reviewed. Data on sex, age at presentation, hospitalization duration, need for ET, hemoglobin (Hb) level, reticulocyte count, direct Coombs test, serum total and indirect bilirubin levels, thyroid function, blood and urine cultures, G6PD status, and blood groups were collected and compared between the G6PD-deficent and G6PD-normal patients.
Results
Of 1,159 NIH patients admitted, 1,129 were included, of whom 646 (57%) were male. Among 1,046 patients tested, 442 (42%) were G6PD deficient, 49 (4%) needed ET, and 11 (1%) had suspected Kernicterus. The G6PD-deficient patients were mainly male (P<0.0001), and had lower Hb levels (P<0.0001) and higher maximum bilirubin levels (P=0.001). More G6PD-deficient patients needed ET (P<0.0001). G6PD deficiency (P=0.006), lower Hb level (P=0.002), lower hematocrit count (P=0.02), higher bilirubin level (P<0.0001), higher maximal bilirubin level (P<0.0001), and positive blood culture result (P<0.0001) were significant risk factors for ET. Maximal bilirubin level was a significant risk factor for kernicterus (P=0.021) and independently related to ET (P=0.03).
Conclusion
G6PD deficiency is an important risk factor for severe NIH. In G6PD-deficent neonates, management of NIH should be hastened to avoid irreversible neurological complications.
Introduction
Neonatal indirect hyperbilirubinemia (NIH) is a common problem among infants12). It affects 60% of full-term and 80% of preterm newborns in the first 3 days of life2). NIH carries a substantial risk for harmful complications which include long-term neurologic impairments and death3). Although significant complications of NIH have become rare in the recent years with therapeutic interventions3), severe NIH secondary to reduced Glucose 6-phosphate dehydrogenase (G6PD) activity is still complicated by kernicterus which is a serious neurological disease34567). G6PD deficiency is an X-linked recessive disease affecting males more than females8). It is common in Bahrain with a prevalence of 22.3%8). The relationship between NIH and G6PD deficiency is well established49). Infants with G6PD deficiency are at higher risk of mortality secondary to bilirubin encephalopathy if total serum bilirubin (TSB) is ≥40 mg/dL3). While spectrums of G6PD-deficient infants in relation to severe NIH have been evaluated in many countries234910111213), to our knowledge it has not been evaluated in Bahrain.
The aim of this study is to identify the prevalence of G6PD deficiency in newborns with NIH requiring phototherapy, to compare patients with reduced G6PD activity and those with normal G6PD activity in terms of level of indirect hyperbilirubinemia, need for exchange transfusion (ET) and development of kernicterus; and to assess risk factors for ET and kernicterus.
Materials and methods
1. Patients and materials
In this case-control retrospective study, all electronic medical records of NIH patients admitted to the Pediatric Department at Salmaniya Medical Complex, Kingdom of Bahrain, at 2 points in times, were reviewed. The first review was from January 2007 till January 2008 and the second review was from December 2008 to June 2010. The reason for this period gap is that during the study period we realized that similar unpublished data were already collected by different investigators in the same institute for the year of 2007; so the decision to combine the patients' data together after checking for similarity was made. All NIH infant admitted to general pediatric wards were reviewed. Patients were excluded from the study if they were missing data about serum bilirubin, had cholestatic jaundice or missing important data required for the study objectives. Readmissions and duplicate registries were also excluded.
Data about sex, gestational age, age at presentation, and length of hospitalization were collected for all patients. Laboratory evaluations including maternal and newborn blood group and rhesus factor (Rh), hemoglobin (Hb) level, reticulocyte count, direct coombs test (DCT), TSB and indirect serum bilirubin (ISB) levels at admission, maximum TSB and ISB levels, serum free thyroxine (T4) and thyroid stimulating hormone level, blood and urine cultures, erythrocyte G6PD status and Hb electrophoresis were gathered. Needs for double volume whole blood ETs were also included. G6PD test was performed using fluorescence spot test (1 mL of whole blood in ethylenediamine tetraacetic acid tube). Kernicterus was suspected in patients who had early neurological symptoms and signs. Radiological imaging such as skull ultrasound, computed tomography scan and magnetic resonance imaging of the brain were reviewed for those patients.
2. Ethical consideration
This study was conducted in accordance with the principles of Helsinki Declaration and it was ethically approved by the secondary care medical research subcommittee, Salmaniya Medical Complex, Ministry of Health, Kingdom of Bahrain.
3. Statistical analysis
The patients' data were analyzed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). The frequencies and percentages were calculated for categorical variables. Chi-square and Fisher exact tests were used to compare categorical variables. Continuous variables were checked for normal distribution using Kolmogorov-Smirnov test. Group data are presented as a mean and standard deviation for normally distributed variables or median and range for nonnormally distributed variables. Due to large sample size, independent Student t test was used to compare continuous variables. G6PD-deficient group was compared with normal-G6PD group in terms of sex, age at presentation, length of hospitalization, Hb level, hematocrit level, reticulocyte count, blood group and Rh incompatibilities, positive DCT, TSB at admission, maximum TSB, need for ET, positive urine and blood cultures, risk for convulsions and suspected kernicterus. These variables were also used to assess the possible risk factors for ET and kernicterus using univariate analysis. Risk factors found to be significant in univariate analysis and have no multicollinearity using a variation inflation factor (VIF) were included in a binary logistic regression to find the independent risk factors of requiring ET or development of kernicterus. The confidence level was set at 95% with 5% confidence interval (CI). P value was considered to be significant if <0.05.
Results
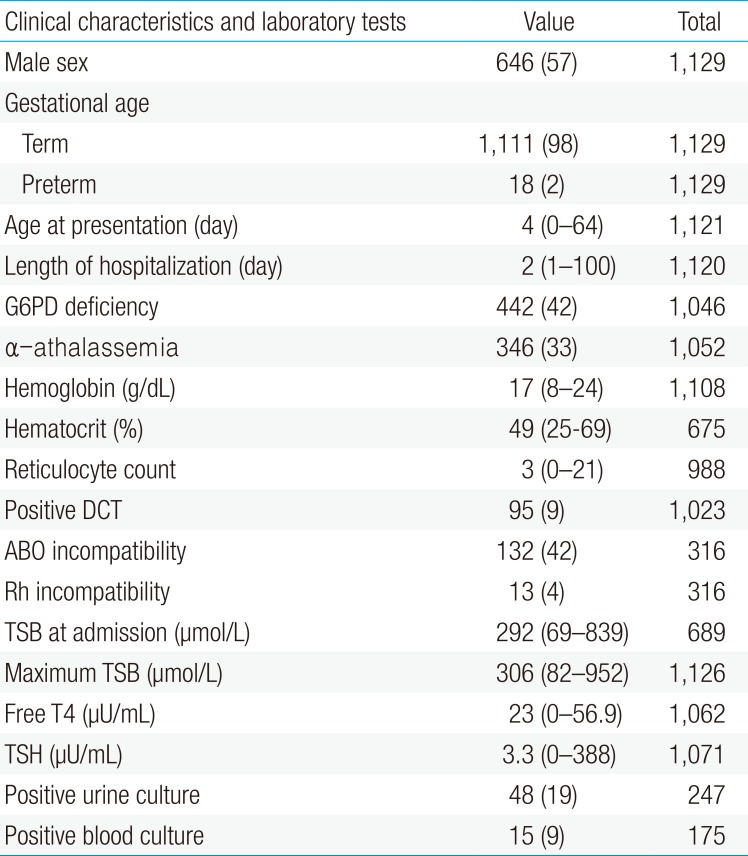
A total of 1,159 admissions were collected in the study periods. Thirty admissions were excluded from the study due to missing data about serum bilirubin in 11 patients, readmissions in 9, duplicate registries in 6; cholestatic jaundice in 3 and 1 patient was excluded due to missing all important data with very low serum bilirubin level (TSB, 97 µmol/L). The remaining 1,129 patients were included. The clinical characteristics and the results of laboratory tests of all newborns included in the study are shown in Table 1. Males were 646 (57%). Out of 1,046 patients tested for G6PD status, 442 (42%) were G6PD-deficient. Hypo and hyperthyroidism were found in 1 patient each. Urine culture was positive in 48 (19%). It grew Escherichia coli in 26 (11%), Klebsiella in 17 (7%), Enterococcus in 2 (1%), Beta-hemolytic streptococci in 2 (1%) and Enterobacter in 1 patient (0.4%). Blood culture was positive in 15 patients (9%). It grew Staphylococcus aureus and E. coli each in 4 patients (2%), Enterococci and beta-hemolytic streptococci each in 3 patients (2%); and Klebsiella in 1 patient (1%).
All the patients received phototherapy. Out of 49 (4%) who had double volume whole blood ET, 10 (1%) required ET twice. Packed red blood cell transfusion was required in 48 (4.3%). Eleven patients (1%) were suspected to have bilirubin encephalopathy (kernicterus). The characteristics of these patients are shown in Table 2. Eight patients (1%) developed convulsions during their admission.
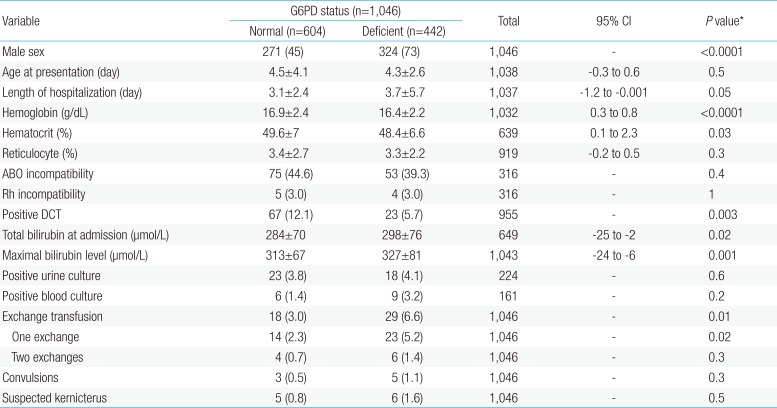
On comparing G6PD-deficient with normal-G6PD neonates (Table 3), males were more affected with G6PD deficiency than females (P<0.0001). G6PD-deficient neonates had lower Hb level (P<0.0001), lower hematocrit (P=0.034), higher TSB at admission (P=0.018), higher maximal serum bilirubin (P=0.001) and higher requirement for ET (P<0.0001). They had longer hospital stay (P=0.05). They also developed more convulsions and suspected kernicterus than normal-G6PD neonates but these were not statistically significant.
In univariate analysis, G6PD deficiency (P=0.006), lower Hb level at admission (P=0.002; 95% CI, 0.4 to 1.8), lower hematocrit level (P=0.02; 95% CI, 0.6 to 6.3), higher TSB at admission (P<0.0001; 95% CI, -206 to -106), higher maximal TSB (P<0.0001; 95% CI, -205 to -131) and positive blood culture (P<0.0001) were statistically significant risk factors for ET (Table 4). The maximal bilirubin level was the only statistically significant risk factor for suspected kernicterus patients (450±172 µmol/L vs. 316±70.5 µmol/L, P=0.02; 95% CI, -243 to -24).

Univariate analysis of the predicted risk factors for the need of exchange transfusion in 1,129 newborns with indirect hyperbilirubinemia
The significant risk factors for ET were tested to determine multicollinearity (defined as a VIF>8) between each other and were put into a logistic regression model. In this model, the maximal TSB level (P=0.03) was found to be independently related to requirement for ET (Table 5).
Discussion
NIH is a multifactorial disease910). Any disease that causes increase in bilirubin production and/or reduces conjugation can lead to NIH210). Hemolytic diseases like ABO incompatibility, Rh incompatibility, spherocytosis and G6PD deficiency can increase bilirubin production while Crigler-Najjar syndrome, hypothyroidism and prematurity can reduce conjugation2). Breast milk feeding may also act as a modifier that predisposes the risk for development of severe NIH with a specific genotype14). Unconjugated bilirubin is mainly produced by erythrocytes turnover15). Hemolysis, which is a frequent cause of NIH, results in increased serum free Hb which is metabolized to produce more bilirubin515).
G6PD is one of the antioxidant enzymes that protect the cell from injury7812). G6PD deficiency is estimated to affect 200 million individuals worldwide9). It is more common in the Mediterranean area, Middle East, India, China, and Africa8). G6PD deficiency is an independent risk factor for high serum bilirubin level ≥18 mg/dL10). It is considered as one of the most common clinically significant enzyme defects489). G6PD-deficient individuals are usually healthy and asymptomatic but acute hemolysis may occur after ingestions of certain drugs, food, exposure to certain chemicals, infections or hypoxia78913). They could be attributable to the early onset hemolysis due to oxidative stress as a result of perinatal events12). Chronic nonspherocytic hemolytic anemia may rarely occur789).
Our study showed that G6PD deficiency is a common problem among NIH infants in a Middle East country like Bahrain. The prevalence of G6PD deficiency in NIH patients also varies in different parts of the world241216). NIH is more common to occur in Asian and African than in European G6PD deficient newborns79). In Turkey, the prevalence of G6PD deficiency is only in 1%–5% of the population with male to female ratio is 3:1 and 3.8% of NIH infants are G6PD deficient4). In our study, we found that 42% of NIH patients were G6PD-deficient which is double compared to general population (22%)8). Similar to other studies37), our study showed that most of G6PD-deficient newborns were males (73%).
A protocol for assessment of NIH should be established in nurseries6). Systematic assessment for risk of severe NIH, close follow up and prompt intervention are important to prevent complications6). There is a high concern that underrecognition and inadequate investigation of severe NIH in otherwise healthy neonates contribute to early readmission and potential long-term complications, including bilirubin-induced encephalopathy and kernicterus17). Every effort should be made to detect newborns at risk of severe NIH before discharge from hospital17). Yellowness of lower extremities, palms and soles noticed on visual assessment by health care workers is significantly associated with a high serum bilirubin level1). In a well-lit room or day light, NIH can be identified by blanching the skin with digital pressure to visualize the underlying color of the skin and subcutaneous tissue6). However, visual assessment of bilirubin level via the degree of jaundice can lead to error, particularly in infants with dark skin6).
Transcutaneous bilirubin (TcB) nomogram is offered as a tool that can help the clinician in the management of NIH18). TcB devices are more accurate than visual inspection, can save time and money and in many times spares neonate from an invasive procedure18). TcB is accepted to be used to diagnose and monitor NIH but it might be less accurate when TSB is rapidly increasing due to the lag time between tissue and serum bilirubin18).
Further investigations like G6PD tests and follow-up planning could be carried out to assess the risk of and to prevent severe NIH if serum bilirubin level was checked at the time of discharge from hospital17). Early detection of G6PD deficiency via newborn screening is feasible, cost effective and allows early prevention of severe NIH and kernicterus8). G6PD deficiency should be confirmed by rapid fluorescent spot test or quantitative spectrophotometry of red blood cell enzyme activity when clinical and hematological findings are suspicious7). G6PD c.563C>T mutation is a significant risk factor for early development and moderately severe NIH11).
In our study, G6PD-deficient neonates were found to have lower Hb and hematocrit levels compared to normal-G6PD neonates. Similar to our study, Moiz et al.11) found significant differences in Hb level and reticulocyte counts in G6PD-deficient compared to normal newborns. Weng and Chiu3) also showed that G6PD-deficient infants had lower Hb level with an evidence of hemolysis. Lower Hb level in G6PD-deficient newborn is associated with higher peak serum TSB level3). Like our study, Atay et al.4) found that TSB at presentation and the maximum level were higher in the G6PD-deficient compared to normal patients. Furthermore, in a case-control study, Kaplan and Abramov13) showed that G6PD-deficient neonates are more prone to develop NIH, require phototherapy and their hematocrit levels are lower. In Ainoon et al. study9), out of 38 G6PD-deficient neonates, 63% had NIH and 28.9% had moderate to severe NIH that required phototherapy or ET. However, Koosha and Rafizadeh2) studied 376 newborns with NIH and showed no statistical differences in the lowest Hb level, reticulocyte count and highest TSB, between G6PD-deficient and normal newborns. Atay et al.4) also found no difference in hematocrit level and reticulocytes count between G6PD-deficient and normal neonates.
NIH secondary to G6PD deficiency is treated like NIH due to other causes7). Phototherapy or ET are the recommended treatment to prevent the development of severe NIH and possibly kernicterus6712). Despite intensive phototherapy, severe NIH may still need ET to prevent bilirubin encephalopathy12). Our study showed that G6PD deficiency is a significant risk factor for the need of ET. In addition, it has been proven that infants with idiopathic NIH showed unexpected rise in serum TSB after ET if the donor blood was G6PD deficient12). Elevated serum bilirubin due to ongoing subclinical hemolysis might lead to phototherapy continuation and possible repeat of ET12). Therefore, donor blood should be screened for G6PD status before using it for ET in countries where G6PD deficiency is prevelant12). This test is not routinely performed in Bahrain.
In this study, G6PD-deficient neonates had significantly higher TSB compared to G6PD-normal newborns. Although rare, significant NIH poses a possible risk for permanent neurological deficit or kernicterus711). G6PD deficiency can cause severe NIH that can lead to kernicterus246). In acute or chronic bilirubin encephalopathy, kernicterus was originally characterized by pathological bilirubin staining of the brainstem nuclei and cerebellum6). Acute toxicity of the basal ganglia and various brainstem nuclei by bilirubin within the first week of life will lead to clinical central nervous system findings of bilirubin encephalopathy6). Kernicterus is a preventable complication6). The resurgence of a largely preventable disease like kernicterus secondary to severe NIH is of a great concern17). The duration of phototherapy can be used as a surrogate marker for the severity of NIH but it can be shortened by ETs10).
In conclusion, G6PD deficiency is a common disease in infants with NIH. It is considered an important risk factor for severe NIH which may result in kernicterus. In infants with G6PD deficiency, management of NIH should be hastened to avoid irreversible neurological complications. Further studies are required to evaluate the relationship between the G6PD enzyme level and the severity of NIH and to set a preventive protocol to avoid the neurological impairment for newborns at risk.
This study was limited by the lack of data about birth weight and by its' retrospective nature. Another limitation is that we may have included G6PD-deficient newborns within the normal group because testing for G6PD may be falsely negative during acute hemolysis12) and in heterozygous female newborns8) where lionization phenomena can occur. In addition, this study was limited by the lack of the exact levels of G6PD enzyme activity for all G6PD-deficient newborns. However, Ainoon et al.9) found no significant association between the level of enzyme activity and the severity of NIH. This study can help the clinicians to screen and allow early treatment for newborns at risk. Early treatment will not only reduce the incidence of severe NIH and kernicterus but also minimize the risks of unintentional harm such as maternal anxiety, reduced breast feeding and unnecessary costs or treatment6).
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.