Clostridium difficile colonization and/or infection during infancy and the risk of childhood allergic diseases
Article information
Abstract
Purpose
The gut microbiota can influence several diseases through immune modulation; however, the exact role of microbes such as Clostridium difficileand the relationship between microbiota colonization and allergic diseases are not well known. This study aimed to determine the relationship between C. difficilecolonization and/or infection (CDCI) during infancy and allergic diseases during early childhood.
Methods
Infants 1–12 months of age presenting changes in bowel habits for more than 2 weeks were enrolled in this study. After dividing them into 2 groups according to the presence and absence of C. difficile, the risk of allergic disease development during childhood was identified and compared.
Results
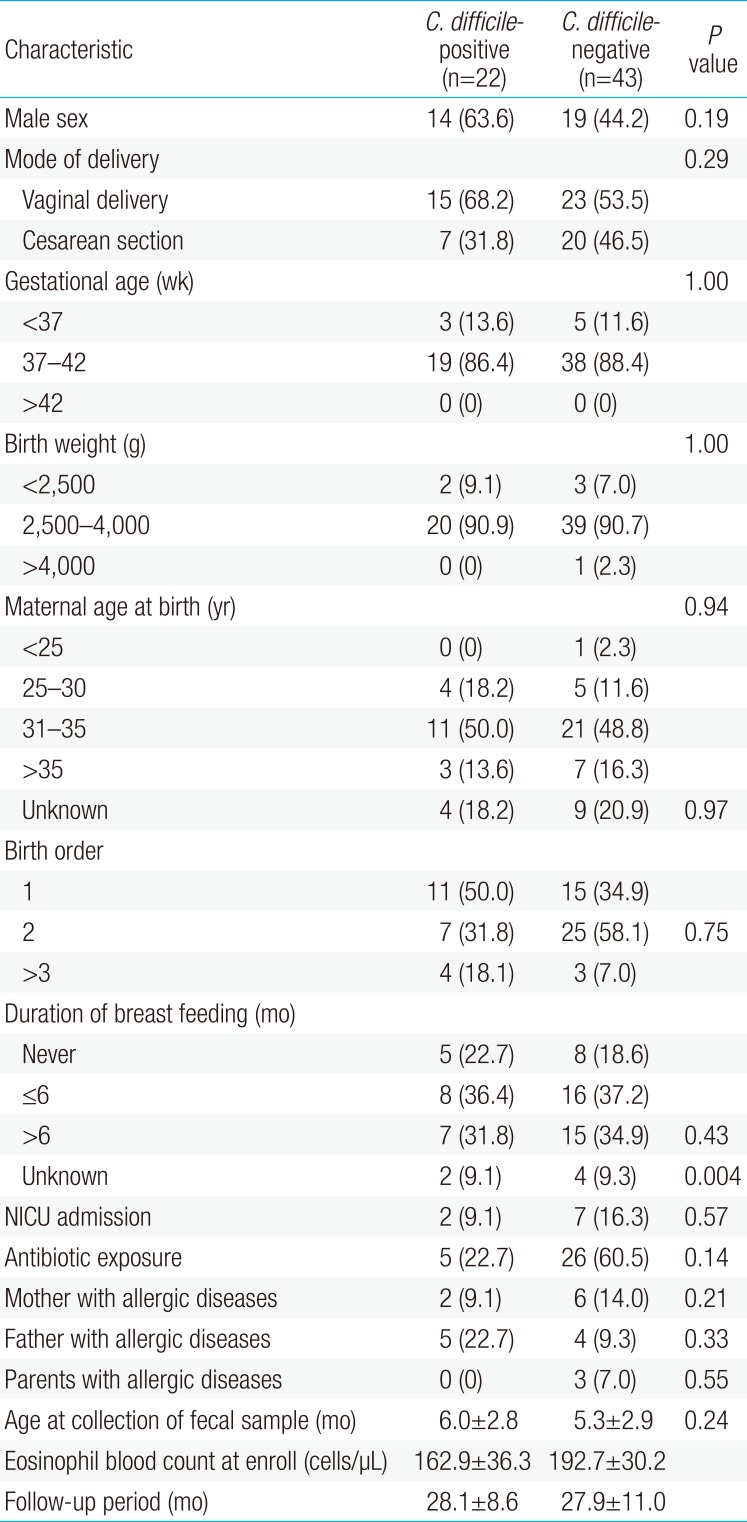
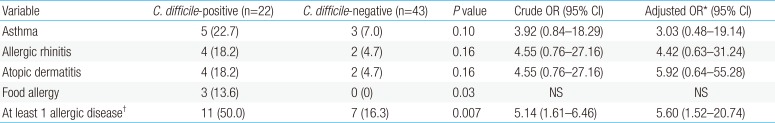
Sixty-five patients were included in this study; 22 (33.8%) were diagnosed with CDCI. No significant differences were observed in baseline characteristics between the C. difficile-positive and -negative groups except for antibiotic exposure (22.7% vs. 60.5%, P=0.004). Compared to the C. difficile-negative group, the risk of developing at least one allergic disease was higher in the C. difficile-positive group after adjusting other variables (adjusted odds ratios, 5.61; 95% confidence interval, 1.52–20.74; P=0.007). Furthermore, food allergies were more prevalent in the C. difficile-positive group (P=0.03).
Conclusion
CDCI during infancy were associated with a higher risk of developing allergic diseases during early childhood. These results suggest that CDCI during infancy might reflect the reduced diversity of the intestinal microbiota, which is associated with an increased risk of allergic sensitization. To identify the underlying mechanism, further investigation and a larger cohort study will be needed.
Introduction
The intestinal microbiota is a key source of immune development and regulation early in life. Deprivation of microbial exposure is thought to predispose to immune dysregulation and the development of atopic disease1). In particular, alterations in microbial exposure and the gastrointestinal microbiota early in life can influence allergic sensitization2345). Hygiene hypothesis has suggested that microbial exposure early in life plays a critical role in developing the immune system, thereby avoiding the development of allergic diseases6). Recent studies have demonstrated that the gastrointestinal microbiota plays a definitive role in atopy development. Colonization of neonatal germ-free mice with a conventional microbiota protected these animals from mucosal invariant natural killer T-cell accumulation7). In addition, B cells isolated from gut-associated lymphoid tissues can induce the development of regulator T cells and alleviate asthmatic symptoms89). Liao et al.10) demonstrated an age-matched response to Toll-like receptor stimulations during the first 2 years of life, thus providing strong evidence for the establishment of immune modulation and maturation in neonates.
Clostridium difficile is a gram-positive, anaerobic, spore-forming rod bacterium that colonizes in 10% to 70% of children younger than 12 months of age11). When the normal colonic microbiota is disrupted, C. difficile can propagate and cause infection12). The overall disease spectrum can range from asymptomatic colonization to severe infection exhibited by severe diarrhea1314). Although specific harmful or protective species of microbes have not yet been identified, there is limited evidence indicating that C. difficile might be one of the microbes associated with atopy5). A recent study has reported that clusters IV and XIVa of genus Clostridium can induce colonic-regulatory T cells for regulating immune responses15). Although the importance of gut bacteria in allergic diseases is undeniable, how individual bacteria species affect allergic disease development is not yet well known. The increasing incidence of atopy might be due to the increasing incidence of community-acquired C. difficile infection in younger children16). The influence of C. difficile colonization and/or infection (CDCI) during infancy after the settlement of normal flora in the gastrointestinal tract on allergic diseases during late childhood remains unknown.
To determine the relationship between CDCI during infancy and the development of allergic diseases during childhood, we hypothesized that CDCI during infancy could alter gut microbiota and increase the risk of development of allergic diseases during early childhood.
Materials and methods
1. Study design and population
Infants 1 to 12 months of age who were admitted to the pediatric gastrointestinal clinic at Gachon University Gil Medical Center between June 2009 and December 2012 were enrolled in this study. These infants had either a change in bowel habits or diarrhea for more than 2 weeks as their main symptom. They underwent laboratory stool tests, including C. difficile testing. Demographic information such as sex, age, mode of delivery, gestational age, birth weight, admission history to a neonatal intensive care unit, duration of breastfeeding, maternal age at delivery, birth order, antibiotic exposure, and eosinophil count were obtained from the medical records and analyzed retrospectively. Antibiotic exposure was defined as positive when the child had ever been administered oral antibiotics for more than 3 consecutive days. No distinction was made between different antibiotics or multiple courses of antibiotics versus a single course. After at least 2 years, the history of allergic disease was collected via parental report questionnaires. Exclusion criteria were as follows: previously confirmed allergic disease, lactose intolerance, documented systemic disease, immune deficiency, immunosuppressive therapy, history of receiving blood products during the prior 3 months, patients who did not undergo stool analysis, records lacking information regarding baseline characteristics, or inability to contact. Lactose intolerance was defined on the basis of a history of improvement in the symptoms of diarrhea after the ingestion of lactose-free formula. All patients were enrolled after fully informed consent was obtained from their parents. This study was approved by the Institutional Review Board of Gachon University Gil Medical Center (approval number: GBIRB2016-163).
2. Microbial analysis
Naturally passed stool samples were collected from the diaper of each infant after defecation. Diagnosis of CDCI were confirmed when C. difficile was identified in the stool culture and/or rapid immunoassay was positive for C. difficile toxins A and B. The C. difficile–positive group included C. difficile colonization and/or infection. The C. difficile–negative group had negative results for both tests. At the same time, rotavirus testing was performed for stool samples. Additionally, a respiratory virus test was performed if patients had respiratory symptoms. Extraction of toxin A and toxin B from C. difficile was conducted using an enzyme-linked fluorescent immunoassay (VIDAS CDAB, BioMerieux SA, Marcy l'Etoile, France). For the C. difficile stool culture, the collected stool was placed onto culture media of ChromID C. difficile agar (BioMerieux SA) and an anaerobic culture was conducted for 48 to 72 hours. Colonies grew black, which contrasted sharply with the clear background of the agar. This enabled easy detection of C. difficile. We used oral metronidazole 20 mg/kg per day for 10 days for toxin-positive cases if there was no improvement using conservative management.
3. Definition of allergic disease outcomes
After at least 2 years, the history of allergic diseases was collected through parent-reported questionnaires. In the survey, a Korean version of the International Study of Asthma and Allergies in Childhood questionnaire17) was used; the characteristics of this survey have been reported in detail elsewhere. Asthma prevalence was determined by lifetime and current (past 12 months) wheezing episodes. We also recorded whether the asthma had been diagnosed by a doctor and whether treatment had occurred in the past 12 months. Similar questions were also asked in regard to atopic dermatitis (AD), allergic rhinitis (AR), and food allergy (FA).
4. Statistical analysis
The chi-square test and Fisher exact test were used to evaluate demographic markers and estimate unadjusted odds ratios (ORs) and relative risks (RRs) with a 95% confidence interval (CI) for the outcomes of asthma, AR, AD, and FA. Logistic regression models were used to estimate adjusted ORs (aORs) with a 95% CI for the outcomes of the C. difficile–negative group and potential confounding effects such as gender, age when the fecal sample was collected, gestational age, birth weight, breastfeeding, and parents with allergic diseases. All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA), P<0.05 was considered significant.
Results
1. Characteristics of the study population
Of the 71 patients, 6 were excluded from the study due to lactose intolerance (n=2), history of AD (n=2), and loss to follow-up (n=2). The remaining 65 infants were divided into 2 groups according to the presence of CDCI. Twenty-two infants (33.8%) were positive for C. difficile and 43 (66.2%) were negative. In the C. difficile–negative group, one patient had rotavirus and 4 patients had a viral respiratory infection (respiratory syncytial virus subgroup B for 3; adenovirus for 1). Other pathogens were not confirmed. The mean age when fecal samples were collected was 5.5±2.9 months. Of the 65 infants, 33 (50.8%) were male (Table 1). Except for antibiotic exposure, no significant differences were observed in the mode of delivery, gestational age, birth weight, maternal age at birth, birth order, duration of breastfeeding, history of neonatal intensive care unit admission, or parental history of allergic diseases between the 2 groups (22.7% vs. 60.5%, P=0.004). The mean follow-up period was 28.0±10.2 months, which was not statistically different between the 2 groups.
2. CDCI and allergic disease outcomes
Between ages 2 and 5 years, 12.3% (8 of 65) of the infants had parent-reported asthma, whereas 9.2% (6 of 65) of the infants had AR. The prevalence of AD was 9.2% (6 of 65) and the prevalence of FA was 4.6% (3 of 65). More infants with CDCI developed at least one allergic disease between ages 2 and 5 years compared to infants without CDCI (P=0.007) (Table 2). Food allergies were more prevalent in the C. difficile–positive group (P=0.03). Two children were allergic to eggs and 1 was allergic to soy. CDCI may be associated with a higher risk of development of at least one allergic disease (aOR, 5.60; 95% CI, 1.52–20.74; Table 2). However, the increased risks of asthma, AR, and AD were not statistically significant.
Discussion
This study demonstrated that the presence of CDCI during infancy was associated with a higher risk of development of at least one allergic disease during childhood. An association between the presence of C. difficile and the development of allergic diseases was not found when allergic diseases were analyzed individually with the exception of FA. Antibiotic exposure was more common in the C. difficile-negative group (P=0.004) (Table 1). These findings may be explained by the results of previous studies showing that the incidence of community-acquired CDCI in infants without antibiotic exposure reached 78% unlike in adults16). Moreover, the results suggest that the environmental sources like food, water, and animals, as well as host factors, may play an important role in community-acquired CDCI, and different strain types and genetic diversity of C. difficile can be affect the diverse sources of CDCI18).
C. difficile in the intestinal microbiota, regardless of the strain, is significantly associated with modification of the composition of the microbial ecosystem14). It is likely that beneficial bacteria are replaced by overgrowth of pathogenic bacteria, thereby reducing the diversity of intestinal flora19). These alterations, such as delayed colonization with beneficial bacteria, might interfere with the development of immunologic tolerance. The absence of a proper immunosuppressive mechanism by regulatory T cells can result in an imbalance between TH1 and TH2 cells and, in turn, cause TH1-mediated and TH2-mediated inflammatory disease5).
Commensal bacteria that normally populate the gastrointestinal tract influence allergic responses to food. In a recent study of the association between altered commensal microbiota and food allergen sensitization, the intestinal microbiota was found to regulate innate lymphoid cell function and intestinal epithelial permeability in mice, and sensitization to food is regulated by these innate mechanisms20). Bisgaard et al.19) found that reduced diversity in the human microbiome is a possible risk factor for life style-related disorders such as atopic disease, possibly through a modifying influence on immune maturation during infancy. Reduced diversity of the intestinal microbiota was causally related to FA in previous studies of the infantile microbiome and allergic disease2122). These findings are similar to those of our study regarding differences in FA between the C. difficile-positive (13.6 %) and C. difficile-negative groups (0%) (P=0.03) (Table 2). We hypothesized that the presence of C. difficile in the intestinal microbiota might possibly reflect the reduced diversity of the intestinal microbiota, which is associated with an increased risk of allergic sensitization. This is in agreement with previous studies2324). However, the mechanisms that change the composition of the intestinal microbiota, which regulates allergic responses to food, are unclear. Therefore, additional research of the association between the actual composition of the gastrointestinal microbiota and the development of FA should focus on the presence of C. difficile.
The association between C. difficile colonization and/or infection and AR has not been reported previously. In the present study, CDCI were not associated with the development of AR. However, several studies have reported the role of probiotics in the treatment of AR2526). Probiotic treatment for AR is associated with lower symptom scores and medication use25), suggesting that there is a relationship between gastrointestinal microbiota and AR.
In our study, CDCI were not associated with the development of AD or asthma. This is partly in agreement with the results of a previous study that intestinal bacterial diversity unrelated with the development of asthma or AD, which was explained by the different genetics27). However, another study reported that colonization by C. difficile at the age of one month was associated with asthma and AD throughout the first 6 to 7 years of life5). Therefore, the influence of the intestinal microbiota on the development of AD and asthma remains controversial, and a large-scale prospective study may be necessary for further investigation.
This is one of the few studies that have shown that CDCI at ages 1 to 12 months after settlement of normal flora in the gastrointestinal tract could influence the development of allergic diseases during early childhood, which might partially explain the effect of the gastrointestinal microbiota on allergic diseases. Because the understanding of the profound influence of commensal microbes on maturation of the immune system has grown, strategies that reduce changes in the composition of the intestinal microbiota may have broad benefits. One such strategy would be cautious use of antibiotics. Several lines of evidence confirm that antibiotic administration can result in gut microbiota dysbiosis, i.e., disturbance in composition and function28). In particular, antibiotic use during infancy strongly perturbs intestinal bacterial populations and has often been cited as a contributing factor to the increasing prevalence of allergic diseases20).
The present study has several limitations. First, this study used stool samples for gut microbiota analysis, which might not reflect the composition of the intestinal tract. Nevertheless, it can be assumed that the dominant microbiota in the intestinal tract should be detectable in the feces by a culture test. Second, because infants enrolled in this study were limited to patients who were admitted to the hospital with specific symptoms, they could not represent the community. Third, the data regarding allergic diseases in children were collected via parental report questionnaires without physical examinations performed by doctors. Therefore, the prevalence rates of allergic diseases might have been overestimated. In addition, the parents answered questions based on their memory, which make responses highly subjective. These responses might have been influenced by recall bias. Fourth, stool tests were performed only once. Therefore, we could not rule out the possibility of CDCI after this study in the C. difficile-negative group. Finally, we did not determine the allergic disease factors, such as vitamin D levels, respiratory virus, and air pollution, in this study.
In conclusion, CDCI might be associated with a higher risk of developing at least one allergic disease. These results suggest the probability of a causal relationship between changes in the intestinal microbiota composition and the development of allergic diseases during early childhood. To confirm the effect of CDCI on the development of allergic diseases, further prospective research involving measurements of serum IgE levels and larger cohorts are required. Furthermore, because the prevalence of allergic diseases usually changes according to age, longer follow-up studies are needed. Finally, more detailed studies are needed to determine how the diverse microbiota could influence immune responses in infants.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.