Effects of low-dose topiramate on language function in children with migraine
Article information
Abstract
Purpose
This study aimed to verify the safety of low-dose topiramate on language development in pediatric patients with migraine.
Methods
Thirty newly diagnosed pediatric patients with migraine who needed topiramate were enrolled and assessed twice with standard language tests, including the Test of Language Problem Solving Abilities (TOPs), Receptive and Expressive Vocabulary Test, Urimal Test of Articulation and Phonology, and computerized speech laboratory analysis. Data were collected before treatment, and topiramate as monotherapy was sustained for at least 3 months. The mean follow-up period was 4.3±2.7 months. The mean topiramate dosage was 0.9 mg/kg/day.
Results
The patient's mean age was 144.1±42.3 months (male-to-female ratio, 9:21). The values of all the language parameters of the TOPs were not changed significantly after the topiramate treatment as follows: Determine cause, from 15.0±4.4 to 15.4±4.8 (P>0.05); making inference, from 17.6±5.6 to 17.5±6.6 (P>0.05); predicting, from 11.5±4.5 to 12.3±4.0 (P>0.05); and total TOPs score, from 44.1± 13.4 to 45.3±13.6 (P>0.05). The total mean length of utterance in words during the test decreased from 44.1±13.4 to 45.3±13.6 (P<0.05). The Receptive and Expressive Vocabulary Test results decreased from 97.7±22.1 to 96.3±19.9 months, and from 81.8±23.4 to 82.3±25.4 months, respectively (P>0.05). In the articulation and phonology validation in both groups, speech pitch and energy were not significant, and all the vowel test results showed no other significant values.
Conclusion
No significant difference was found in the language-speaking ability between the patients; however, the number of vocabularies used decreased. Therefore, topiramate should be used cautiously for children with migraine.
Introduction
Migraine is one of the most serious primary headaches in children and occurs in up to 10.6 % of children aged 5–15 years1). Migraine attacks may have negative effects on every aspect of a child's quality of life, including daily activities, interactions with peers, and family dynamics23). Therefore, many children with migraine who do not tolerate to conservative treatments or to nonsteroidal anti-inflammatory drugs are appropriate patients for prophylactic therapy.
Calcium channel blockers, β-blockers, serotonin antagonists, antidepressants, antihistamine mimetics, and antiepileptic drugs have been prescribed for migraine prophylaxis. Among these drugs, antiepileptic drugs, especially topiramate (TPM), are most frequently prescribed for children and adolescents. However, TPM should be used cautiously, particularly in pediatric patients, because TPM has several side effects: weight loss, hypohidrosis, paresthesia, dizziness, kidney dysfunction, ataxia, acute myopia, and secondary angle closure glaucoma. TPM also can cause cognitive dysfunction, including language disturbances in patients with epilepsy when administered at the recommended dose45678). Several reports suggest that low dose TPM may lead to language disturbances in adult patients with migraine91011). However, there are no published reports investigating the effects of TPM on migraine in children. Therefore, this study aimed to verify the safety of low-dose TPM on language function in pediatric patients with migraine.
Materials and methods
1. Patients
Ninety-six pediatric patients diagnosed with migraine who needed TPM for migraine prevention because of the frequency more than 4 times per week were enrolled at the Department of Pediatrics of Chonbuk National University Hospital from May, 2011 to January, 2016. All patients received TPM monotherapy and were excluded when taking any medications which altered language function during follow-up. Brain magnetic resonance imaging and electroencephalography were examined to rule out secondary headaches and standard language tests were performed 2 times at minimum, at least 3 months apart. 30 patients were enrolled for this study after 66 patients were excluded. 58 patients excluded because of incomplete data and eight patients who needed other prophylactic medications for migraine were excluded. Bilingual patients were not included.
2. Methods
All patients were assessed at least twice using standard language tests: the Test of Language Problem Solving Abilities (TOPs), a Korean version of the Receptive & Expressive Vocabulary Test (KREVT), Urimal Test of Articulation And Phonology for the articulation screening test, and the computerized speech lab 4500 (CSL Model 4500, Kay Elemetric 2004, Lincoln Park, NJ, USA) for speech analysis (voice onset time, total duration, vowel formant [F1, F2], pitch and energy).
The first language test was administered just prior to TPM treatment and the second test after at least 3 months of TPM therapy. We used the Korean versions of the language tests because all the patients exclusively spoke Korean. The average follow-up period was 4.3±2.7 months.
The starting dose of TPM was 25 mg/day at bed time, and then was increased up to 100 mg/day or 1 mg/kg/day. The mean dosage was 0.9 mg/kg/day.
This study was performed with approval from the Institutional Review Board of Chonbuk National University Research Council (CUH 2014-07-008).
3. Language tests
1) Test of Language Problem Solving Abilities
TOPs is an evaluation tool that measures metalinguistic skills of transforming logical thinking to language in children between the ages of 5 and 12 years. The illustrations used in this test were developed by the Seoul Community Rehabilitation Center, Republic of Korea1213).
The TOPS is divided into 3 categories and contains a total of 17 illustrated materials. The first category consists of 18 questions about determining cause, including interrogative, “Why” questions (determining cause). The second category consists of 20 questions about problem solving, including “How” questions (making inference).
Finally, the third category consists of 12 questions about making predictions, including answers to questions such as “How do you know?” and “What happens?” (making predictions). Scores ranging from 0 to 2 were assigned depending on responses to each category, with a top score of 100. Answers were recorded and documented immediately after the tests were completed. Scores were defined as raw scores, mean scores, and total scores for each category. The length of articulation for each answer was compared using the mean length of utterance in words (MLU-w), which is defined as the mean score of the length of articulation obtained by adding all the words in the answer divided by the number of sentences included in the answer13).
2) Korean version of the Receptive & Expressive Vocabulary Test
The Receptive & Expressive Vocabulary Test (REVT) is a standardized test that is approved for evaluation of receptive and expressive vocabulary development and is applicable to children from 2 years old to adult14). Raw scores were calculated based on basal and ceiling results, and equivalent ages were also measured 14).
3) Urimal test of articulation and phonology
The Urimal test of articulation and phonology (U-TAP; Urimal means Korean language) is a test designed for individuals with abnormal articulation that have a systematic approach in solving articulation problems by examining their pronunciation in a single vocabulary or in a sentence. The test, designed for patients from 2 to 12 years old, presents a certain picture to the children and leads them to make a sentence which includes a targeted phoneme. Accuracy is calculated by dividing the number of incorrect phonems by the total number of phonems and is expressed as percent correct15).
4) CSL analysis
The computerized speech laboratory (CSL) is an acoustic analysis system with robust hardware for data acquisition, complemented by a versatile suite of software available for speech analysis, teaching, research, voice measurement, clinical feedback, acoustic phonetics, and forensic work. The equipment allows for the simultaneous capture of microphonic and laryngographic signals. The individual is asked (after an adequate training) to emit a sustained /a/,/e/,/i/,/o/,/u/ and a short sentence. The standard deviations of the intensity and frequency of the sentence (expressed as the percentage of to the mean value) were obtained.
4. Statistical analysis
Statistical significance was determined analyzed using IBM SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Paired t tests were used to compare differences before and after TPM treatment. All values are expressed as mean±standard deviation. The value P<0.05 was considered significant.
Results
The mean age of patients was 144.1±42.3 months (male:female ratio=1:2.3).
Patients did not change drugs, and completed all follow-up language tests during the study period.
Twenty patients responded well to the treatment, and patients' migraine was reduced after taking TPM. There were 5 patients who showed no significant differences in in headache frequency or headache-related disability. In 5 patients, the migraine was aggravated. Among of these 5 patients, 3 patients had a medication change after the second language test was completed.
1. Results of the TOPS
The language parameters of TOPs were changed after TPM treatment; however, it was not statistically significant. The highest score in the “Determine cause” category was 22. There was no significant difference between baseline (15.0±4.4) and TPM treatment scores (15.4±4.8, P>0.05) (Table 1). The highest score in the “making inference” category was 30. The mean score at baseline was 17.7±5.6 and 17.6±6.6 after TPM treatment; this difference was also not significant (P>0.05) (Table 1).
The highest score in the “Predicting” category was 20. The mean score of 11.5±4.5 was obtained for pediatric migraine patients before TPM treatment, which increased to 12.3±4.0 after taking TPM. Moreover, this value was not significant (Table 1) (P> 0.05).
Total score of TOPs which differences before and after TPM monotherapy was not significant (Table 1) (P>0.05). Of a maximum score of 100, the mean score of patients before taking TPM was 44.1±13.5, which increased to 45.3±13.6 after taking TPM.
2. MLU-w in TOPS
The total mean length of utterance in words (MLU-w) during the test decreased from 5.5±2.0 to 4.9±1.5 after TPM treatment (Table 2) (P<0.05).
The difference in MLU-w for the “determine cause” category of questions before and after TPM treatment was significant, decreasing from 5.5±1.7 to 4.3±1.3 (Table 2) (P<0.05).
With respect to “making inference” the mean MLU-w score decreased 6.2±2.1 to 4.6±1.5 (P<0.05) after taking TPM. However, the “predicting” category of the mean MLU-w score decreased from 5.5±2.3 to 4.3±1.8 after TPM treatment; however, it was not significant (Table 2) (P>0.05).
3. Results of the REVT
REVT did not change significantly. In the Receptive Vocabulary Test, the mean score of 97.8±22.1 was obtained for patients with pediatric migraine before TPM treatment, which decreased to 96.3±20.0 after taking TPM. In addition, Expressive Vocabulary Test was 81.8±23.4 to 82.3±25.4 months. In the control group, the Receptive Vocabulary Test was 112.5±17.9 and Expressive Vocabulary Test was 94.1±9.3 (P>0.05).
4. Result of U-TAP
U-TAP has not been shown any specific change. When we compare the groups premedication and postmedication, there was no damage found in articulation and phonology validation in both groups and control group which have 100%.
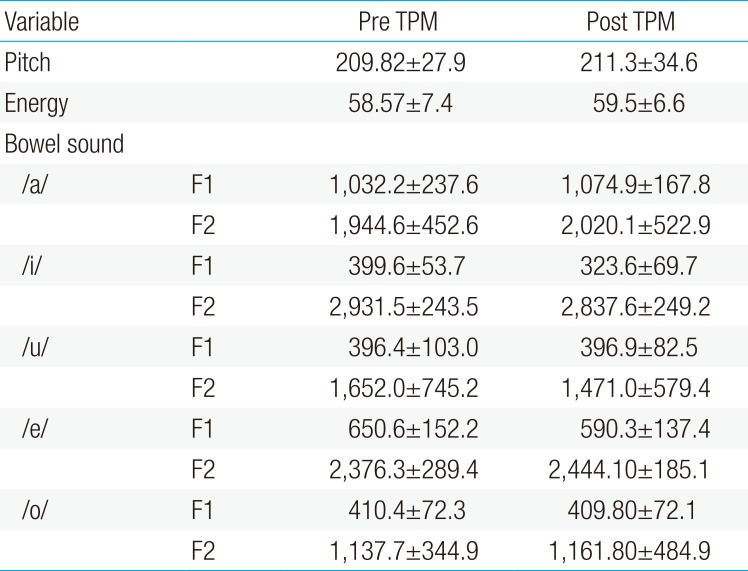
The mean score of pitch test increased from 209.8±27.9 to 211.3 ±34.6 after TPM treatment (P>0.05). In the energy test, the mean score of 58.6±7.4 was obtained for pediatric patients with migraine before TPM treatment, which increased to 59.5±6.6 after taking TPM. For parameter pitch and energy, there was no significant influence on the data from the measuring between the groups. There was no corresponding significant influence of medication for both pitch and energy.
5. Result of CSL analysis
In addition, for parameter vowel (in Korean /a/,/i/,/u/,/e/,/o/), there was no significant influence on the data obtained from the groups. These results did not yield a significant correlation between the data of both before and after medication of vowel (Table 3) (P>0.05).
Discussion
Although TPM was initially developed for the treatment of epilepsy, it is an effective migraine preventive therapy in adults, as demonstrated in several studies1617). The doses of TPM required to treat migraine are lower than those needed to treat epilepsy, resulting in a lower prevalence of adverse effects18). Treatment options for pediatric migraine are particularly limited, and there are few migraine preventive agents approved for use in children 1920). Thus, preventive pharmacologic treatments for migraine in children need to be based on efficacy and safety. Precisely how TPM prevents migraine is unclear; however, generally, it appears to reduce the genetically-derived headache that provokes migraine attacks in susceptible individuals.
Fallah et al.21) showed TPM as a safe and effective drug for pediatric migraine prophylaxis, which reduces monthly frequency, severity, duration, and disability of migraine in children. Other studies have shown that TPM at 100 mg/day is effective in the prevention of migraine headaches and in reducing the severity of the attack222324). Similarly, the present study showed that TPM is effective in the reducing migraine. In contrast, several studies have reported language impairments such as deterioration of verbal expression, verbal learning, work memory, and verbal fluency with TPM treatment25262728). In our study, we analyzed the effects of TPM on language development using a set of specific language evaluation tools. Pediatric patients with migraine with no history of anticonvulsant medication were evaluated on the basis of language characteristics before and after TPM medication using TOPs, MLU-w, REVT, U-TAP, and CSL prospective analysis throughout the study.
The TOPs was used to examine problem-solving ability, which refers to the ability to comprehend the causes of events, speculate on conditions, and solve problems13). There was no significant difference in language problem-solving skills before and after TPM treatment. The mean “determine cause” score declined in 13 of 30 patients (43.3%) after TPM, the mean “making inference” score declined in 16 of 30 patients (53.3%), and the mean “predicting” score was reduced in 14 of 30 patients (46.6%). In the MLU-w portion of the TOPs, the mean “determine cause” score decreased from 5.5 to 4.3, the mean “making inference” score decreased from 6.2 to 4.6, and the mean “predicting” score decreased from 5.5 to 4.3 following TPM treatment. Similarly, the total problem-solving MLU-w score decreased from 5.5 to 4.9 after TPM treatment. These results show that TPM treatment may cause vocabulary function impairment in problem solving and deterioration of speaking sufficient sentences. After TPM medication, the answers of the examinees appeared to become more ambiguous, were phrased in shorter sentences, and were delayed because of difficulties in word choice selection. Furthermore, improper grammar was used in some cases. This suggests that attention is required when TPM is used in pediatric migraine patients.
The REVT was used as a tool for comparative analysis of receptive vocabulary development skills, which encompasses the ability to see, hear, and understand linguistic stimuli13). Receptive vocabulary test and expressive vocabulary test were compared before and after TPM monotherapy in our study. Although 12 of 30 patients' score was decreased in the receptive vocabulary test and 8 of 30 patients' score was decreased in the expressive vocabulary test, REVT was not significant.
In addition, the results of U-TAP remained unchanged. In CSL analysis, all correlations were not significant.
This study has several limitations. First, this study lacked language evaluation tools. Second, it was conducted at a single center with a smaller subject group. Finally, larger studies are required to evaluate the long-term efficacy, optimal dose, length of treatment, and long-term effects of TPM. Future studies could be considered with positive effects of language development after reductions in migraine headache.
In conclusion, our results show that TPM is effective in the prevention of migraine headache and in reducing the severity of the attack. Furthermore, we found no difference with the ability of language problems between patients; however, still there is deterioration in the number of the vocabularies they use. Therefore, it is recommended to use TPM while considering the side effects of language in pediatric patients with migraine even at low doses.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.