Acute pancreatitis in hand, foot and mouth disease caused by Coxsackievirus A16: case report
Article information
Abstract
Coxsackievirus A16 (CA16), which primarily causes hand, foot, and mouth disease (HFMD), is associated with complications, such as encephalitis, acute flaccid paralysis, myocarditis, pericarditis, and shock. However, no case of pancreatitis associated with CA16 has been reported in children. We report a case of CA16-associated acute pancreatitis in a 3-year-old girl with HFMD. She was admitted because of poor oral intake and high fever for 1 day. Maculopapular rashes on both hands and feet and multiple vesicles on the soft palate were observed on physical examination. She was treated conservatively with intravenous fluids. On the fourth hospital day, she had severe abdominal pain and vomiting. The serum levels of amylase and lipase were remarkably elevated (amylase, 1,902 IU/L; reference range, 28–100 IU/L; lipase, >1,500 IU/L; reference range, 13–60 IU/L), and ultrasonography showed diffuse swelling of the pancreas with a small amount of ascites. The real-time reverse transcription polymerase chain reaction result from a stool sample was positive for CA16. CA16 can cause acute pancreatitis, and should be considered in the differential diagnosis of abdominal pain in children with HFMD.
Introduction
Coxsackievirus A16 (CA16) belongs to the genus Enterovirus of the family Picornaviridae. Enterovirus is one of the most common and important human pathogens. Following oral or respiratory acquisition, initial replication occurs in the pharynx and intestine. Clinical infection occurs if replication proceeds in the reticuloendothelial system and the virus spreads via a secondary, sustained viremia to target organs such as the central nervous system, heart, and skin. Tropism to the target organ is determined in part by the serotypes. CA16, which primarily causes hand, foot and mouth disease (HFMD), is rarely associated with complications such as encephalitis, acute flaccid paralysis, myocarditis, pericarditis, and shock1). However, no case of pancreatitis associated with CA16 has been reported in children yet. Here, we report a case of CA16-associated pancreatitis in a 3-year-old girl with HFMD.
Case report
A 3-year-old girl visited the Emergency Department because she had not been able to eat well and had a fever since one day ago. She had no abdominal pain, vomiting, or diarrhea. She had taken only the prescribed acetaminophen one day before. According to her past medical history, she went to kindergarten, had not traveled recently, had no history of pancreatitis, autoimmune disease, choledochal cyst, or pancreatic ductal abnormalities, and her family history was negative for pancreatic disease. She has one older sister, and her sister did not have symptoms such as fever. Her vital signs at admission were as follows: blood pressure, 97/60 mmHg; heart rate, 104 beats/min; respiration rate, 24 breaths/min; and body temperature, 37.4℃. Her height was 98 cm (50th–75th percentile), and her weight was 15 kg (50th–75th percentile). On physical examination, multiple vesicles on the soft palate and maculopapular rashes were observed on both the hands and feet. She had a clear breathing sound, regular heartbeat without murmur, and soft abdomen with a normoactive bowel sound without an abdominal mass or abdominal tenderness. In initial laboratory studies, the serum glucose was decreased (41 mg/dL), erythrocyte sedimentation rate was slightly increased (17 mm/hr; reference range, 0–10 mm/hr), and C-reactive protein was slightly elevated (12.9 mg/L; reference range, 0.0–5.0 mg/L), and other laboratory studies were within the normal ranges. The white blood cell count was 7.8×103/µL, hemoglobin was 12.6 g/dL, platelet count was 252×103/µL, blood urea nitrogen was 15.0 mg/dL, creatinine was 0.42 mg/dL, cholesterol was 187 mg/dL, albumin was 4.3 g/dL, total bilirubin was 0.39 mg/dL, direct bilirubin was 0.11 mg/dL, aspartate aminotransferase was 34 IU/L, alanine aminotransaminase was 10 IU/L, sodium was 135 mmol/L, potassium was 4.4 mmol/L. Amylase and lipase tests were not performed at the time of admission. Urine ketone body was 3 positive in urinalysis. A chest X-ray showed no active lung lesions, cardiopulmonary angle blunting, or cardiomegaly. Abdomen X-rays showed a nonspecific bowel gas pattern without ileus or pancreatic calcification.
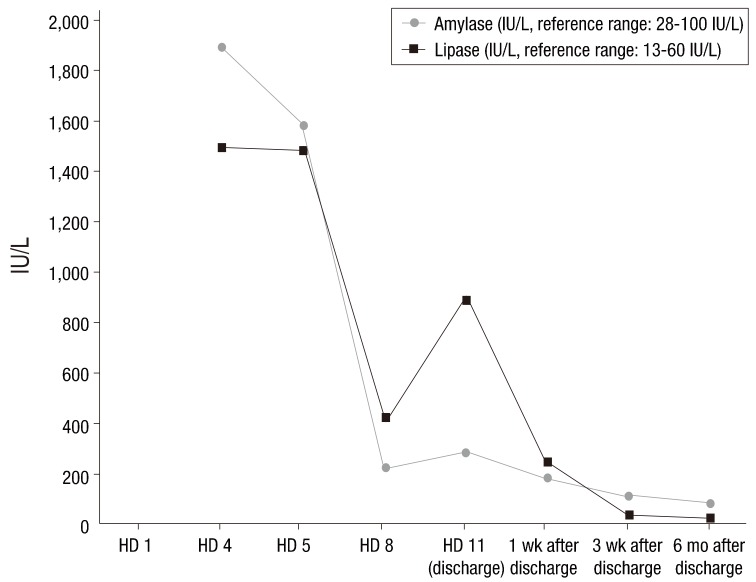
We treated the child conservatively with intravenous fluid. On the first hospital day (HD), her fever subsided. On the third HD, she complained of ear pain caused by acute otitis media and intravenous amoxicillin/clavulanate was administered at a dose of 90 mg/kg/day. On the fourth HD, she experienced severe epigastric pain and vomiting. Her vital signs were as follows: blood pressure, 107/80 mmHg; heart rate, 109 beats/min.; respiration rate, 24 breaths/min; and body temperature, 37.2℃. On physical examination, she complained of epigastric tenderness. The serum levels of amylase and lipase were remarkably elevated (amylase, 1,902 IU/L; reference range, 28–100 IU/L; lipase, >1,500 IU/L; reference range, 13–60 IU/L). Ultrasonography of the abdomen showed mild diffuse swelling of the pancreas from head to tail with a small amount of ascites (Fig. 1), which suggested acute pancreatitis.
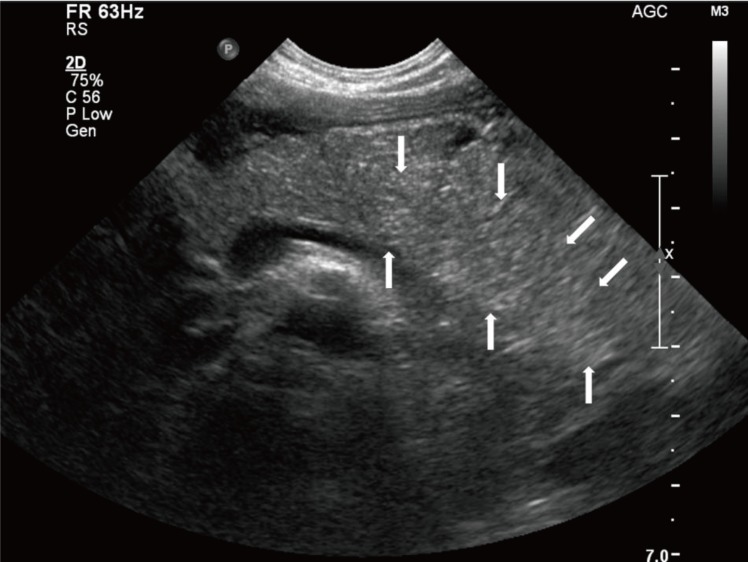
Abdominal ultrasonography performed on the fourth hospital day showed mild diffuse swelling of the entire pancreas, with a small amount of ascites (white arrows). There were no significant findings in the liver, visible spleen, gall bladder, and kidneys, and no evidence of common bile duct dilatation.
Considering the patient's history of HFMD, we thought that enterovirus, which is a common cause of HFMD, could be a pathogen. We asked the Korea Centers for Disease Control and Prevention for enterovirus typing of her stool sample. In the first step, real-time reverse transcription polymerase chain reaction (RT-PCR) was performed on the specimens targeting the 5' noncoding region capable of detecting all 68 serotypes, and the result was enterovirus-positive. In the second step, 2-step RT-PCR was performed targeting viral protein 1, which has a different structural enterovirus protein for each subtype, and CA16 was detected in the sample.
After acute pancreatitis was diagnosed, the patient was fasted and given sufficient intravenous fluid. Acetaminophen and ibuprofen were given for pain control. Serum amylase and lipase were serially followed (Fig. 2). The fever, epigastric pain, and vomiting were recovered on the sixth HD. She was discharged on the 11th HD without pancreatitis-related complications. On the day of discharge, the serum levels of amylase and lipase were remarkably reduced (amylase, 289 IU/L; lipase, 893 IU/L).
She was followed up after discharge without any symptoms, and pancreatitis has not recurred yet. Laboratory tests performed 6 months after discharge showed normal levels of serum amylase and lipase (Fig. 1).
Discussion
We introduced a case of pancreatitis secondary to HFMD associated with CA16 infection. This is the first report of CA16-associated acute pancreatitis in a child to our knowledge.
Because she took a reasonable quantity of acetaminophen before the diagnosis of pancreatitis, it was thought that the possibility of pancreatitis due to the medication was low. She had no history of trauma or recurrent pancreatitis or familial history of pancreatic disease. Therefore, we thought that the possibility of genetic and congenital diseases, such as anomalous union of pancreaticobiliary duct, was low. Above all, we thought that CA16 infection was the main cause of pancreatitis when we considered the fact that pancreatitis occurred shortly after the onset of HFMD.
For confirmation, detection of the virus directly in the pancreatic tissue is the obvious method. Because she was young and the CA16 was detected in the stool specimen, invasive diagnosis, such as endoscopic ultrasound-guided fine needle aspiration biopsy, was not performed.
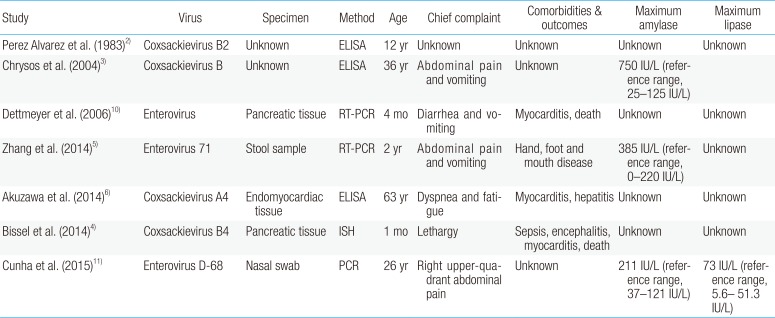
Coxsackievirus group A is well known as the most common pathogen of HFMD. Coxsackievirus group A infection usually has mild symptoms and a good prognosis. Enteroviruses, such as coxsackievirus group B and enterovirus 71, are reported to be common causes of acute pancreatitis2345). In China, a 2-year-old girl was admitted because of acute abdominal pain and vomiting for 2 days and diagnosed with acute pancreatitis. She had a fever and maculopapular rash on her hands and feet 4 days before admission. According to RT-PCR in a stool sample, enterovirus 71 was positive. She was conservatively treated and discharged without complications on the eighth HD. Some cases of enterovirus-associated acute pancreatitis have been accompanied with life-threatening diseases, such as myocarditis and encephalitis, and patients can even die (Table 1).
In adults, one case of coxsackievirus group A-associated acute pancreatitis with myocarditis and hepatitis has been reported in Japan6). The patient was a 63-year-old man who was admitted due to dyspnea. He showed pulmonary congestion, a complete atrioventricular block, left ventricular dysfunction and elevated serum troponin I and liver enzyme levels. Acute pancreatitis occurred 5 days after admission. A high antibody titer against coxsackievirus A4 was revealed.
The pathogenesis of enterovirus-induced pancreatitis is not well known, but it has been reported that the virus can damage the target tissue through a series of mechanisms that cause autoimmunity to an organ-specific antigen caused by virus infection7). Depending on its tropism, the virus can directly damage the target tissue, such as the pancreas, and produce a self-antigen. The self-antigen causes a series of reactions of dendritic cell, T cell, and B cell to release auto-antibodies. Autoantibodies activate the immune complex-mediated complements, damaging the target organ. In addition, acute damage from viral infection can cause innate immune cells such as macrophages to produce excessive inflammatory cytokines and damage target tissues. Therefore, CA16 is thought to induce pancreatitis through the above mechanism.
Some viral infections, such as coxsackievirus B, cytomegalovirus, and varicella-zoster virus can cause diabetes mellitus by destroying the beta cells of the island of Langerhans89). Our case had no symptoms associated with diabetes mellitus, such as polyuria, polydipsia, and polyphagia. The serum levels of glucose remained within the normal range, and the result of a hemoglobin A1c test performed 6 months after discharge was 5.1%. Diabetes mellitus can develop after pancreatitis as a late complication; therefore, regular monitoring of diabetes mellitus-related symptoms and signs is needed.
Coxsackievirus group A infection usually has mild symptoms and a good prognosis, but can have severe symptoms and a poor prognosis6). Therefore, patients with HFMD should be monitored carefully, and when the patient complains of abdominal symptoms, acute pancreatitis should be considered in differential diagnosis.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.