Is vaginal reflux associated with urinary tract infection in female children under the age of 36 months?
Article information
Abstract
Purpose
To determine the relationship between vaginal reflux (VR) and urinary tract infection (UTI) in female children aged <36 months.
Methods
A single center retrospective study was performed for 191 girls aged <36 months, with a diagnosis of febrile UTI, who underwent a voiding cystourethrography (VCUG) for assessment of vesicoureteral reflux (VUR) at Sanggye Paik Hospital. Fifty-one girls, who underwent VCUG for assessment of congenital hydronephrosis or renal pelvis dilatation, without a UTI, formed the control group. The correlation between the presence and grade of VR and UTI was evaluated.
Results
The prevalence rate of VR was higher in the UTI (42.9%) than control (13.7%) group (P<0.05), with a higher VR severity grade in the UTI (mean, 0.64) than control (mean, 0.18) group (P<0.05). On subanalysis with age-matching (UTI group: n=126, age, 5.28±2.13 months; control group: n=22, age, 4.79±2.40 months; P=0.33), both VR prevalence (43.65% vs. 18.18%, P<0.05) and grade (0.65 vs. 0.22, P<0.05) remained higher in the UTI than control group. Presence and higher grade of VR were associated with UTI recurrence (P<0.05). VR was correlated to urosepsis (P<0.05). The renal defect rate of patients with VR (VR [+]/VUR [+]) was not different from that of patients without VR (74% vs. 52%, P=0.143) in the VUR group; however, it was higher than that of VR (+)/VUR (−) patients (74% vs. 32%, P=0.001). If a child with VR (+)/VUR (+) is exposed to a UTI, the risk of renal defect increases.
Conclusion
Occurrence of VR is associated with UTI recurrence and urosepsis in pediatric female patients.
Introduction
Urinary tract infection (UTI) is one of the most common causes of bacterial infections in childhood, with an incidence rate of 8.4% in girls and 1.7% in boys under the age of 6 years.123) Recurrent UTI can lead to irreversible damage to the renal parenchyma, resulting in hypertension and chronic renal failure. As such, UTI recurrence is considered as an important cause of end stage renal disease. Of significance, about 30%–50% of patients experience recurrence after a first UTI.14) The vesicoureteral reflux (VUR) has been recognized as a risk factor for renal parenchymal damage56) and a weaker predictor of renal scarring than UTI recurrence.7)
In contrast, the presence of vaginal reflux (VR) in female children is largely considered as being a transient functional alteration of the genitourinary system, due to the age-dependent relative position of the urethra and vagina, and, therefore, as a cause of false positive results of urine culture in the diagnosis of UTIs.789) As such, VR is not recognized as a risk factor for UTI in the same way that sex, age, race, circumcision status in boys, and voiding dysfunction are.101112) Moreover, the probability of VR as a contributing factor of UTI and UTI recurrence is still considered to be arguably small.913)
However, considering the anatomical structure of the female genitalia, VR could present an opportunity for ascending infection via a shared mechanism of urinary stasis with other known risk factors associated with UTI or recurrent UTI. The main purpose of our study was to clarify whether VR is a contributing factor to the occurrence of UTIs in girls, and to determine if any relationship between VR and UTI was influenced by the severity of the VR.
Materials and methods
1. Patients
We retrospectively reviewed the medical records of 235 girls under the age of 3 years who underwent a voiding cystourethrograhy (VCUG) in the pediatrics department of Inje University Sanggye Paik Hospital (Seoul, Korea), between January 2000 and May 2016. Patients diagnosed with febrile UTI between January 2000 and December 2014 underwent assessment with renal sonography, 99mtechnetium- -labeled dimercaptosuccinic acid (DMSA) scan, and VCUG as nonselective intervention (n=210). VCUG was selectively performed for patients in whom abnormalities were identified on ultrasonography or DMSA scan, as well as those with recurrent UTI after 2015 (n=25). These 235 cases were screened to exclude patients with an incomplete or missing medical record (as some children had first been evaluated at another hospital; n=27) and patients with significant renal anomaly, such as renal agenesis, duplicated kidney and polycystic kidney (n=17). Therefore, 191 cases of febrile UTI were enrolled into the study. Another 51 patients were enrolled into the control group who underwent VCUG for evaluation of sonographic screening for renal anomaly, but had no history of febrile UTI. The results of renal ultrasonography of the control group are as follows: pelvic dilatation (n=32), hydronephrosis (n=7), multicystic dysplastic kidney disease (n=5), double renal pelvis (n=4), extrarenal pelvis (n=1), screening for imperforated anus combined anomaly (n=1), nephrocalcinosis (n=1). Exclusion criteria for the control group were the same as for the UTI group.
This study was approved by the Institutional Ethics Review Board of the Inje University Sanggye Paik Hospital (2017-02-024), and the requirement for informed consent was waived.
2. Definition
Febrile UTI was defined based on previously published criteria, as follows.81415): a tympanic fever >38℃, for children 2–36 months old, or a rectal fever >37.5℃, for children 0–2 months old, and bacteriuria confirmed by urine culture. A positive urine culture was defined using the following modified criteria1416): any growth of a pathogenic organism in a suprapubic tap; or a colony-forming-unit count ≥104 or a count of a single organism ≥105/mL in samples obtained by catheterization or midstream of voided urine in toilet trained girls.
A recurrence of UTI was also defined based on previously published criteria,313) as a second positive urine culture result at >1 month after the resolution of a first UTI, using the same criteria previously described for a UTI, in addition to fever or urinary symptoms and signs (foul odor urine, dysuria). Variables associated with UTI recurrence were dichotomized as negative or positive.
Patients with a diagnosis of febrile UTI were all successfully treated using casual antibiotic treatment. Medical imaging, including renal ultrasound (US), DMSA renal scanning and VCUG, was performed to confirm the presence of a VUR. The following data were collected for analysis: age (months) at first VCUG, VCUG results (US findings, DMSA scan results), length of hospital stay, duration of fever (days), results of blood test obtained at the initial assessment (white blood cell [WBC] count and C-reactive protein [CRP]), results of urinalysis analysis (nitrite level, urine WBC count, urine red blood cell count [RBC]), urine protein level, and body height and weight. Age, length of hospital stay, duration of fever, WBC, and CRP were evaluated as continuous variables. US findings were classified as positive when the following features were identified: change in parenchymal echogenicity, renal swelling, thickened pelvic wall, and loss of the corticomedullary differentiation. On DMSA, findings of single or multiple cortical defects and either a focal or diffuse decreased uptake pattern in one kidney were defined as positive. The severity of VUR was graded based on the system of the International Reflux Study Group,317) with a grade ≥1 in one or both kidneys defined as a positive finding.
Urinalysis results were recorded as bifurcated nominal variables, as follows: nitrite and proteinuria were identified based on their presence or absence in laboratory results, with positive pyuria and hematuria defined as a WBC and RBC, respectively, >4 on high power field. Obesity was defined based on body weight and height which exceeds 95% of the normative values on the 2007 Korean National Growth charts.18)
3. Measurement of the severity of VR
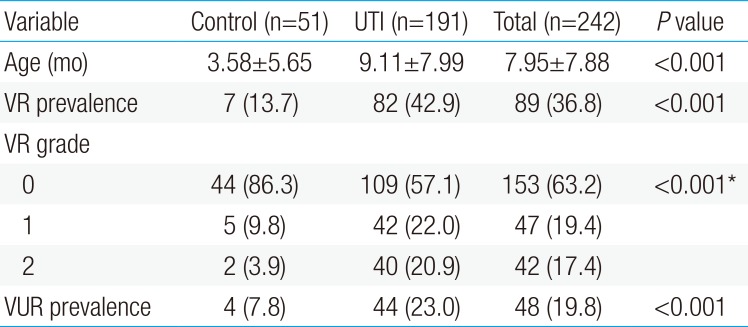
The severity of VR was evaluated using the modified imaginary scoring system described by Kelalis et al.,8) with the three grades of hydrocolpos described as follows (Fig. 1): grade 0, no visible VR; grade 1, presence of a tracking flow through the vaginal canal, without vaginal bulging; and grade 2, presence of a tracking flow through the vaginal canal, with observable vaginal bulging.
Vaginal reflux (VR) is classified into 3 grades, based on the modified imaginary scoring system from Kelalis et al.8) 1973. (A) Grade 0, no visible flow of VR; (B) grade 1, presence of a tracking flow through the vaginal canal, without vaginal bulging; (C) grade 2, presence of a tracking flow through the vaginal canal, with detection of vaginal bulging.
4. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). Continuous variables were described as a mean±standard deviation, with differences between the UTI and control group evaluated using a chi-square test, independent sample t test or Mann-Whitney U test, as appropriate for the distribution of the data set. Categorical variables were described as a percentage value, with between-group differences evaluated using a chi-square analysis. Between-group differences in the VR grade were evaluated using a Mann-Whitney U test. A P value<0.05 was considered significant for all tests.
For patients in the febrile UTI group, a univariate Spearman correlation analysis was performed to identify the clinical manifestations and patient-specific characteristics associated with VR, followed by a logistic linear regression analysis to identify predictive factors of VR. The prevalence rate of renal defects associated with VR, identified by DMSA, was also compared between patients with and without VUR using a chi-square analysis.
Results
1. Prevalence rate and severity of VR in our study group
The mean age for the UTI group was 9.11±7.99 months compared to 3.58±5.65 months for the control group (P<0.05) (Table 1). The prevalence rate of VR was 42.9% (82 of 191) in the UTI group and 13.7% (7 of 51) in the control group (P<0.05). The mean VR grade was 0.64 for the UTI group and 0.18 for the control group (P<0.05) (Tables 1, 2). There was no correlation between age and VR prevalence in either group (Table 2).
To control for any effect of age, a subanalysis was performed including only patients 2 to 10 months old (Table 3). The age distribution on this subanalysis was comparable for both groups: UTI, n=126, age 5.28±2.13 months; control, n=22, age 4.79±2.40; P=0.33. The prevalence rate of VR remained higher in the UTI (43.7%) than control group (18.2%) (P=0.024), as did the severity grade of VR (0.65 vs.0.22, respectively, P=0.021).
2. Clinical characteristics according to the presence of VR
The occurrence of VR did not correlate with the prevalence rate of VUR, abnormal US findings and renal defect on DMSA (P>0.05), or with laboratory findings. VR, however, was significantly correlated to UTI recurrence and urosepsis (P<0.05) (Table 4). In contrast, the VR grade correlated with UTI recurrence (P=0.013) but not urosepsis (P=0.147). The incidence rate of VUR also correlated with UTI recurrence (P=0.004).
We conducted multivariate logistic regression analyses, one with VUR as the independent variable and the other with VR grade. The odd's ratio, OR (95% confidence interval [CI]) of UTI for each model was as follows: VUR, OR, 3.06, 95% CI, 1.45–6.47, P=0.003; VR grade, OR, 1.73; 95% CI, 1.15–2.61; P=0.009 (Table 5). The prevalence rate of renal defect among patients with a VUR and VR (74 %) was higher than among those with a VUR but no VR (52%), but with this difference being nonsignificant (P=0.143). No association between VR and the prevalence rate of renal defect was identified among patients in the UTI group only, with or without a VUR (Fig. 2). In the presence of VR, the prevalence rate of a renal defect increased from 32% (without a VUR) to 74% (with a VUR), indicating that the risk of renal defect increases if a child with VR and VUR is diagnosed with a UTI (P=0.001).

Multivariate logistic regression analysis to determine predictive VCUG score and VR grade for UTI recurrence
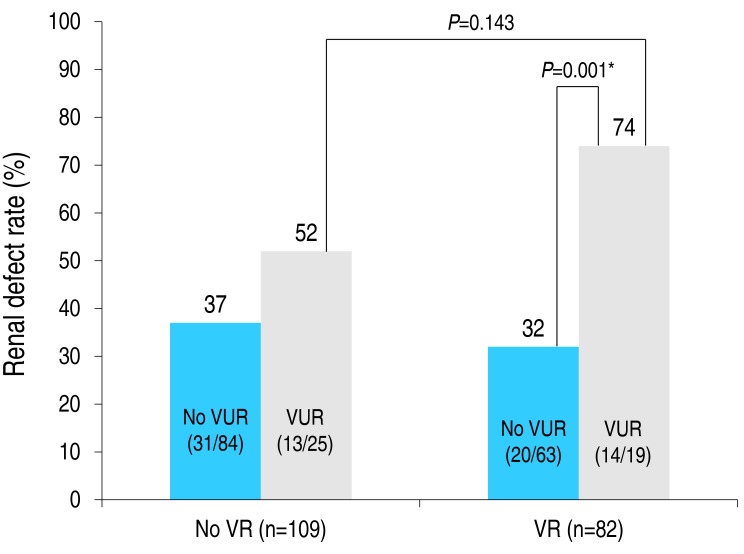
Prevalence rate of renal defect according to the status of vaginal reflux (VR) and vesicoureteral reflux (VUR). Between-group comparisons were performed using a chi-square test, with results as follows: P=0.177 (VR [−] without VUR vs. VR [−] with VUR), P=0.516 (VR [−] without VUR vs. VR [+] without VUR), P=0.143 (VR [−] with VUR vs. VR [+] with VUR), P= 0.001 (VR [+] without VUR vs. VR [+] with VUR). *P<0.05. Odds ratio (95% CI) of renal defect in patients with a VUR: VR vs. no VR group, 1.85 (0.75–4.51) vs. 6.02 (1.90–19.02). Odds ratio (95% CI) of renal defect in patients with a VR: no VUR vs. VUR group, 0.79 (0.40–1.567) vs. 6.02 (0.71–9.37).
Discussion
A UTI is a common bacterial infection in children that can cause permanent damage to the kidneys. Although a VUR is identified as the most important risk factor for UTIs in current clinical guidelines, other individual risk factors could contribute.1920) A long-term follow-up study (median, 23 years) in Sweden21) reported a higher prevalence of renal damage among females with a pediatric history of UTI, with the recurrence rate of UTI and of renal scarring being higher among those with a VUR than without reflux. Of note, the recurrence of symptomatic UTI persisted after resolution of VUR, with the severity of renal scarring being correlated with the recurrence rate of UTI. This finding is supported by Larcombe22) who reported that damage to the renal parenchymal was strongly correlated with recurrent UTI and with a first UTI at <2 years of age.
In order to prevent permanent renal damage, identification and proper management of recurrent UTI is needed.62324) Known risk factors for recurrent UTIs include: female sex, noncircumcised male, Caucasian race, younger age, voiding dysfunction, obstructive uropathy, wiping from back to front in girls, urination habit, bubble bath, postbowel movement, a first UTI, tight clothing, constipation, neuropathic bladder, sexual activity, and pregnancy.252627) With the exception of immunological and genetic susceptibility to UTIs, risk factors for UTIs all provide opportunity for an ascending infection.2628)
The process of VR during the urinary process was well described by Davis and Chumley29) in 1966. The urination process begins with a contraction of the bladder. As the bladder pressure rises, the sphincter of the ureter relaxes and the urine stored in the bladder escapes through the ureter. The urine exiting the urethra is temporarily trapped inside the pelvis. Although the majority of urine is discharged from the perineal region,30) some urine is infused into the vaginal canal, via the vaginal opening, in proportion to the urinary pressure and urethro-vaginal angle. The presence of an obstructive anatomical structure in the region anterior to the urethra and to labia increases the risk of vaginal infusion during urination.
VR is generally considered as a nonpathologic condition in girls, such as phimosis in boys, as it typically disappears with a change in position (25%) and with age (prevalence rate of VR is 6 fold higher in girls <3 years of age than in those >14 years). Previous studies have also identified VR as a cause of false positive results of urine culture,789) regarding VR as an insignificant factor in UTI etiology, despite the fact that the recurrence rate of UTI is relatively higher among girls with VR than in those without (P=0.087).13)
It has been proposed that VR is a form of bladder bowel dysfunction (BBD) and, as such, would inherently be associated with a greater risk for UTI.3) In urology and gynecology, VR is treated as a cause of voiding dysfunction,3132) assessed using postvoiding US to identify the extent of hydrocolpus induced by VR and to determine the best therapeutic approach to manage urinary incontinence.1031)
Previous studies have reported a prevalence rate of VR of 12.4% to 62%.8929) This large range may reflect differences in the age distribution of study groups, as well as differences in the prevalence of UTI and voiding dysfunction among the study groups. The overall prevalence rate in our study was 36.8%, with a significantly higher rate among the UTI group (43.7%) than the control group (18.2 %). This higher prevalence of VR among patients with a UTI was maintained on subanalysis after age-matching between the UTI and control groups. The higher prevalence rate of VR in the UTI group did not correlate with either obesity or VUR, which is in agreement with most of the previous research9101532) but not all.13) Therefore, based on our findings and previous reports, VR may be a factor associated with recurrent UTI. It has been reported that VR may disappear with age and a change in posture during urination.9) We did not identify an effect of age on the prevalence of VR in our study. However, due to the young age of children included in our study group (<36 months), it was not possible to clearly evaluate the effects of functional change in posture and age on the incidence rate of VR. Moreover, only 3 patients with a VUR underwent follow-up assessment using conventional VCUG. In these 3 patients, the presence of VR and the VR grade remained unchanged at a mean follow-up of 1.3 years. As well, only 18 of the 44 patients with a VUR underwent a follow-up assessment using radioisotope VCUG. Therefore, we were unable to evaluate to evaluate to rate of disappearance of VR with increasing age.
As previously studied, VR has been classified as a type of BBD,33) with the presence of a BBD and any degree of VUR being highly correlated to UTI recurrence.23) In our study, we did not evaluate the relationship between the prevalence rate of VR and the occurrence of BBD. However, we did show that VR was highly correlated with the rate of recurrence of UTI, with the recurrence rate of UTI being significantly higher among girls with a grade 2 VR (vaginal bulging).
In our study, girls with VR in the UTI group had a higher incidence of urosepsis (6%, 5 of 82) compared to those without VR. The mean age of 5 children with urosepsis and VR was 5.58 months. Among these 5 cases, Escherichia coli was cultured in the urine and blood specimens in 4 cases and Enterobacter cloacae in the other. Two of these children only had a VR, with the other 3 having both a VR and VUR. Considering the small number of children with urospesis, our data are descriptive in nature and, therefore, it is uncertain how VR causes urosepsis, regardless of VUR, in female infants.
Renal scarring is more prevalent in patients with a UTI and VUR.2434) In our study, we further described a significant correlation between the rate of renal defect and the presence of VUR among children with a UTI (OR, 2.99; 95% CI, 1.49–5.99; P=0.002). The prevalence rate and VR grade were not significantly related with the occurrence rate of VUR in the UTI group. The renal defect rate in patients with a VUR and VR (VUR [+]/VR [+]) was 73.7%, and higher than the 52.0% rate among VUR [+] patients without VR (VUR [+]/VR [−]), although this association did not reach significance (P=0.143). In the presence of VR, the rate of renal defect was higher among patients with a VUR (74%) than those without a VUR (32%; P=0.001). Therefore, if a child with VR and a VUR develops a UTI, the risk of renal defect increases.
Our study has several limitations which should be acknowledged. Foremost is the retrospective nature of our study which precludes identification of causation, as well as having the possibility of a selection bias. UTI recurrence data was not systematically collected for all cases, and there was no comparison of rates of UTI, UTI recurrence, and VUR to boys.
Despite these limitations, our study presents data to support a possible role of VR in UTI occurrence and UTI recurrence among girls. This evidence has not been previously published, with studies considering VR as a nonpathological transient condition.789) In fact, we propose that VR could be a specific inducing factor of urosepsis in female infants, and a cause UTI recurrence regardless of VUR. Future, prospective systemized studies are needed to clarify the hidden pathologic meaning and reassessment of VR. Further studies should be performed to verify the validity and usefulness of the grading system of VR used in this study.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.