Association between vitamin D and urinary tract infection in children
Article information
Abstract
Purpose
The present study aimed to determine the relationship between serum 25-hydroxyvitamin D (25(OH)D) level and Urinary tract infections (UTIs) in children.
Methods
In this case-control study, 70 children with UTI (case group) were compared with 70 healthy children (control group) in terms of serum 25(OH)D levels. The children were between 1 month and 12 years of age. Serum 25(OH)D levels were measured using enzyme-linked immunosorbent assay (ELISA). The results were analyzed and compared between both groups.
Results
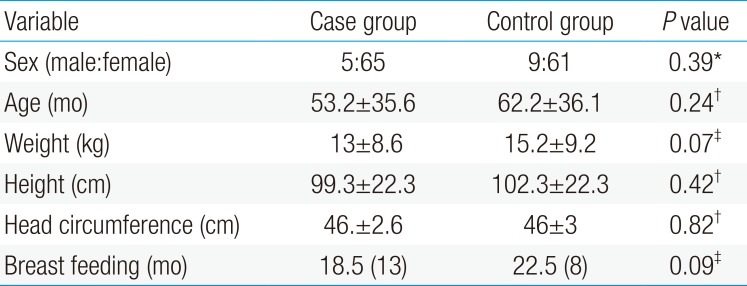
Among 70 children with UTI (case group), 5 children (7.2%) were male and 65 (92.8%) were female. Among the healthy children (control group), 9 (12.8%) and 61 children (87.2%) were male and female, respectively (P=0.39). The mean±standard deviation of age in the case and control groups were 53.2±35.6 and 36.1±60.2 months, respectively (P=0.24). The mean level of serum 25(OH)D in the case group was significantly higher than that of the control group (20.4±8.6 ng/mL vs. 16.9±7.4 ng/mL, P=0.01).
Conclusion
This study showed that there was a relationship between serum 25(OH)D levels and UTI in children. It seems that 25(OH)D plays a role in the pathogenesis of UTI.
Introduction
Urinary tract infection (UTI) is one of the most common infectious diseases in children. The prevalence of the disease in boys and girls is 1% and 5.3%, respectively. The disease appears in 3 forms of cystitis, acute pyelonephritis, and asymptomatic bacteriuria. The most severe form of disease is acute pyelonephritis that is caused by invasion of pathogenic microorganisms to renal parenchyma. Although various microorganisms can cause UTI, Escherichia coli is the most common cause of disease in 80%–90% of cases.123) Delayed diagnosis and treatment will lead to severe diseases such as renal scarring, hypertension and chronic renal failure.4567) Several risk factors such as vesicoureteral reflux predispose the patients for UTI.8) The question has been raised whether vitamin D is a risk factor for UTI. There are some limited and contradictory studies in this field.91011) Some believe that vitamin D deficiency predisposes the patients to have UTI and administration of vitamin D can prevent UTI.910) In contrast, Katikaneni et al.11) believes that administration of vitamin D supplements increases the risk of UTI.
Vitamin D is a secosteroid hormone which is mainly produced in the skin after exposure to ultraviolet radiation.12) Studies show that vitamin D, in addition to calcium-phosphate homeostasis and bone metabolism, has multiple extra skeletal proprieties.13) Immunomodulatory and antibacterial properties are 2 extra skeletal effects of vitamin D.1415) Several studies have been conducted on the role of vitamin D in some infectious diseases including tuberculosis, lower respiratory tract infections (such as pneumonia), and upper respiratory tract infections(such as tonsillitis and otitis).161718) Considering the importance of recognizing the risk factors associated with UTI and preventing serious complications, the present study was performed to determine the relationship between serum 25-hydroxyvitamin D (25(OH)D) levels and UTI in children.
Materials and methods
1. Study participants
In this case-control study, 70 children with UTI (case group) were compared with 70 healthy children (control group) in terms of serum 25(OH)D levels. The study was conducted in Qazvin Children Hospital affiliated to Qazvin University of Medical Sciences. This hospital is the only pediatric referral hospital in Qazvin province.
2. Study design
The age of children was between 1 month and 12 years old. Inclusion criteria for case group included: The first UTI, existence of symptoms for UTI such as fever, poor feeding, poor appetite, vomiting, malaise, abdominal and flank pain, dysuria, frequency; abnormal urinalysis such as pyuria (more than 5 leukocytes per microscopic field) and positive nitrite test; positive urine culture (more than 105 colony forming unit [CFU]/mL of a single pathogen in a midstream urine sample or clean catch method or 104 CFU/mL of a single pathogen via urinary catheterization, or presence of any number of colonies of an organism in urine culture taken by suprapubic method) and lack of known risk factors (such as vesicoureteral reflux, abnormalities of the urinary system including hydronephrosis, urethral stricture into bladder, neurogenic bladder, posterior urethral valves and labial adhesion) and circumcised boys.12) Children with more than once attack of UTI, received antibiotics before admission, underlying disease, and well-known risk factors for UTI, were excluded. Group matching was applied to select 70 healthy children (control group) who presented to the hospital for vaccination or elective surgeries such as tonsillectomy. Consecutive sampling continued until the desired sample size was reached. The 2 groups were matched for age, sex, height, weight, head circumference (up to 3 years), adequate nutrition during breastfeeding (at least for 6 months), social and economic situation (the ratio of family members to bedrooms was used as a measure of socioeconomic situation), average income and family size.1920) Both groups lived in Qazvin and all children were under control and care of health centers until 2 years and received vitamin D regularly. Ultrasonography and voiding cystourethrogram were performed to rule out abnormalities of the urinary system and vesicoureteral reflux. Labial adhesion was excluded by clinical examination. Dimercaptosuccinic acid (DMSA) renal scan (as the gold standard) was used to distinguish between acute lower UTI (cystitis) and acute pyelonephritis. Acute pyelonephritis was confirmed by observing focal or diffuse areas of diminished uptake associated with preservation of renal cortical outline in DMSA renal scan.2)
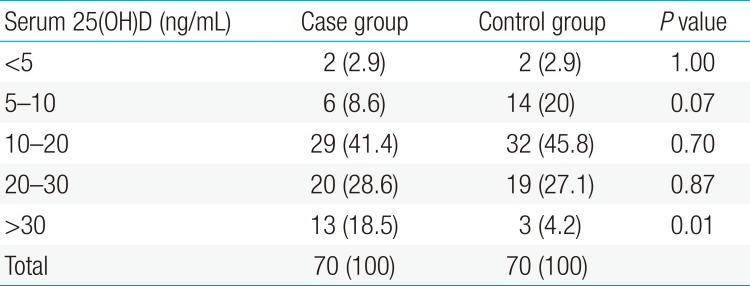
After approval of the plan by the Research Ethics Committee of the University the plan was first introduced to parents. After obtaining parental written consent form and verbal consent from older children, 3 mL of blood was taken from peripheral vein to measure serum 25(OH)D. After centrifugation, serums were isolated from samples and were kept at 20℃ until performing the tests. Serum 25(OH)D test was performed based on the ELISA method using EUROIMMUN kit (No. EQ 6411-9601, Mediziische Labordiagnostika AG Company, Lübeck, Germany). Based on serum 25(OH)D levels, children were divided into 5 groups: less than 5 ng/mL (very severe vitamin D deficiency), 5–10 ng/mL (severe vitamin D deficiency), 10–20 ng/mL (vitamin D deficiency) , 20–30 ng/mL (vitamin D level lower than the optimal level) and 30–50 ng/mL (optimal vitamin D level).21) The tests were performed in Pars laboratory in Qazvin.
3. Statistics
The results were presented in the form of statistical tables and numeric indicators. Chi-square test, t test and Mann-Whitney U test were applied to analyze the obtained data. All analyses were performed with SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). P value of <0.05 was considered statistically significant.
4. Ethics statement
The ethics committee of the Research Department in the Qazvin University of Medical Sciences Qazvin, Iran approved the study (project number: 296). All parents were provided information regarding the research method in simple language. The children were included in the study after their parents agreed and signed the informed consent form.
Results
Among 70 children with UTI (case group), 5 children (7.2%) were male and 65 (92.8%) were female. In healthy children (control group), 9 (12.8%) and 61 children (87.2%) were male and female, respectively (P=0.399). The mean±standard deviation (SD) of age in the case and control groups were 53.2±35.6 and 36.1±60.2 months, respectively (P=0.24). There was no significant difference between 2 groups in terms of sex, age, weight, height, head circumference and duration of breastfeeding (P>0.05) (Table 1). The minimum and maximum of serum 25(OH)D levels in the case group were 8.5 and 42, respectively, with the mean±SD of 20.4±8.6 ng/mL. The minimum and maximum of serum 25(OH)D levels in the control group were 4.3 and 34.4, respectively, with the mean±SD of 16.9±7.4 ng/mL. There was a significant difference between 2 groups in terms of serum 25(OH)D levels (P=0.01). The severity of 25(OH)D deficiencies in case and control groups is shown in Table 2. There was not any correlation between serum 25(OH)D level and inflammatory and non inflammatory markers such as body temperature, white blood cell counts and duration of disease (P>0.05) (Table 3). No significant differences were observed between acute pyelonephritis and cystitis groups in terms of serum 25(OH)D levels (23.9±10.6 ng/mL vs. 20±8.3 ng/mL) (P=0.97) (Table 4). The most common clinical symptoms in children with UTI were fever, malaise and dysuria. E.coli was the most common grown bacteria in UTI patients.

Correlation analysis between serum 25-hydroxyvitamin D (25(OH)D) level and inflammatory and noninflammatory variables in the case group
Discussion
The present study revealed that there is a correlation between serum 25(OH)D levels and UTI in children. There are limited studies on the role of vitamin D in children with UTI. The study conducted by of Nseir et al.22) on 93 patients with a history of recurrent UTI has shown that serum 25(OH)D levels in patients with recurrent UTI are significantly lower than the control group. These authors indicated that vitamin D deficiency is a risk factor for recurrent UTI. Nielsen et al.23) study on 50 patients with UTI and 53 healthy people (control group) showed that there is a significant relationship between urine cathelicidin (LL-37) level and incidence of UTI. The authors concluded that the concentration of LL-37 in the urinary tract system and low susceptibility to LL-37 can raise the probability of UTI in a complex interaction between host and pathogen attributes. The study conducted by Kwon et al.24) on 410 patients showed that vitamin D deficiency is an independent risk factor for UTI after kidney transplantation. They concluded that, physician can predict the incidence of infectious complications after kidney transplantation by measuring serum 25(OH)D level. They suggested that the antibacterial role of vitamin D is associated with the production of antibacterial peptides such as cathelicidin and modulate, β-defensin on cytokines production, and suppressing inflammation.24) Also, the study performed by Tekin et al.25) on 82 children with 2–18 years with first UTI showed that vitamin D deficiency is a risk factor for UTI. In addition, the Yang et al.9) study on 132 infants between 1 to 12 months with first UTI and 106 healthy infants (control group) showed that serum 25(OH)D levels in the case group were significantly lower than the control group. They showed that the incidence of UTI in the group receiving vitamin D supplementation was less than the other group. They concluded that the risk of UTI is high in children with vitamin D deficiency. Another study conducted on 36 children with UTI and 38 healthy children indicated that children with vitamin D deficiency are not able to increase their urine cathelicidin level during UTI. They concluded that vitamin D can prevent the occurrence of UTI using increasing mechanism of urine cathelicidin level.26) It was reported that cathelicidin stimulated the production of chemokines and cytokines by different cells and provided the integrity of the urinary system.272829303132) It is believed that when there is a vitamin D deficiency, macrophages infected with bacterial agents (such as gram-negative bacteria) are not able to produce adequate antibacterial peptides. Failure to produce these peptides predisposes the human to UTI and also increases the severity of the disease.3334)
Contrary to the mentioned studies, study of Katikaneni et al.11) has shown that vitamin D supplementation increases the risk of UTI in formula-fed infants up to 76%. The study of Katikaneni et al.11) was conducted on 315 infants under 3 months old. These authors suggested that administration of vitamin D to 0–3 month old infants should be done with caution. They did not measure serum 25(OH)D level. In our study, the mean serum 25(OH)D level in children with UTI was significantly higher than healthy children. Based on the elimination of confounding variables in our study and the results obtained by Katikaneni et al.11), this question is raised that: how vitamin D administration can lead to UTI? It is believed that supplementation vitamin D can increase the risk of UTI with several mechanisms. Creation of a slight nephrocalcinosis due to administration of vitamin D is one of these mechanisms. Nephrocalcinosis is an excellent context for bacterial growth. 25(OH)D is an immune modulator which has a tendency to decrease immune responses.35) Since the conversion of 25(OH)D to 1, 25-dihydroxy vitamin D is locally done over the infected area, vitamin D prescription results to high production of 25(OH)D. This phenomena results in greater inhibition of the immune system and also, increased risk and aggravation of UTI. As well, 25(OH)D is an antagonist to 1, 25-dihydroxy vitamin D at the vitamin D receptor, and overload of 25(OH)D level could therefore result in an unregulated hyperactive immune response to infection. Theses mechanisms could be answerable for the augmented UTI risk seen by vitamin D administration.11)
This study had some limitations. The sample size was small and this project was a cross-sectional study. Also, we could not measure the serum 25(OH)D levels after completion of treatment. Overall, our data indicate that high level of vitamin D may be a risk factor of UTI in children, thus we recommend further investigations in this regard.
Acknowledgments
Our thanks and best regards go to Research Department of Qazvin University of Medical Sciences and parents of children for their corporations.
Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.