Targeted busulfan and fludarabine-based conditioning for bone marrow transplantation in chronic granulomatous disease
Article information
Abstract
Chronic granulomatous disease (CGD) is a primary immunodeficiency disease caused by impaired phagocytic function. Hematopoietic stem cell transplantation (HSCT) is a definitive cure for CGD; however, the use of HSCT is limited because of associated problems, including transplantation-related mortality and engraftment failure. We report a case of a patient with CGD who underwent successful HSCT following a targeted busulfan and fludarabine reduced-toxicity myeloablative conditioning. Intravenous busulfan was administered once daily for 4 consecutive days (days –8 to –5), and the target area under the curve was 75,000 µg·hr/L. Fludarabine (40 mg/m2) was administered once daily for 6 consecutive days from days –8 to –3. Antithymocyte globulin (2.5 mg/kg/day) was administered from days –4 to –2. The patient underwent successful engraftment and did not have any severe toxicity related to the transplantation. Conditioning with a targeted busulfan and fludarabine regimen could provide a better outcome for HSCT in CGD, with close regulation of the busulfan dose.
Introduction
Chronic granulomatous disease (CGD) is a primary immunodeficiency disease caused by impaired phagocytic function. Although allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative option in patients with CGD, HSCT is challenging due to the high risk of graft rejection and transplantation-related mortality. Therefore, a reduced toxicity myeloablative conditioning with delicate regulation of drug administration can offer a better solution for successful HSCT. Here, we report a case of a CGD patient who underwent HSCT using a reduced-toxicity myeloablative conditioning regimen with fludarabine and targeted busulfan assisted by therapeutic drug monitoring (TDM).
Case report
A 10-month-old boy with a history of recurrent infections including multiple lymphadenopathies, streptococcal sepsis, and perianal abscess visited the outpatient clinic of Seoul National University Children's Hospital. Subsequent to this initial visit, he was admitted three times for the treatment of bacterial and fungal pneumonia. The nitroblue tetrazolium reduction test was negative, and the neutrophil dihydrorhodamine (DHR) oxidation test showed an oxidized peak of 0%, indicating a lack of function of the nicotinamide dinucleotide phosphate (NADPH) oxidase complex. The results of the father's and mother's DHR oxidization tests showed oxidation peaks of 100% and 35%, respectively. Given these results, the patient was diagnosed to be X-linked CGD, determined by the identification of a carrier mother. A nonsense mutation of the CYBB gene (nucleotide 481 C>T) was found at the patient.
At 4 years of age, the patient underwent related bone marrow transplantation from a full matched sibling donor (younger brother). Bone marrow transplantation was performed with informed consent. The DHR test of the donor showed normal NADPH function (99.8% oxidized peak). The conditioning regimen consisted of targeted busulfan, fludarabine and rabbit antithymocyte globulin (ATG). Intravenous busulfan was given over 3 hours once daily for 4 consecutive days at days –8 to –5. On the first day, the patient received 120 mg/m2 of busulfan, and targeted dose of busulfan was administered on subsequent three days. The target area under curve (AUC) of busulfan was 18,500–19,000 µg·hr/L, and the target cumulative AUC of busulfan for the 4 days was 75,000 µg·hr/L1). The total cumulative AUC of busulfan that was administered over 4 days was 73,148.1 µg·hr/L, with a daily AUC of busulfan of 22,043.7 µg·hr/L, 21,250.2 µg·hr/L, 19,691.5 µg·hr/L, and 10,162.7 µg·hr/L. Fludarabine (40 mg/m2) was given once daily for 6 consecutive days on days –8 to –3. ATG (2.5 mg/kg) was given once daily on days –4 to –2. Graft versus host disease (GVHD) prophylaxis consisted of cyclosporin A and methylprednisolone. Other supportive care was given according to the guidelines for HSCT of our center2).
Neutrophil engraftment of absolute neutrophil count over 500/µL and 1,000/µL were both achieved at 11 days after HSCT. At 18 and 22 days after HSCT, platelet counts over 20,000/µL and 100,000/µL, respectively, were achieved. Chimerism analysis revealed that 47.74% of cells were recipient cells at 14 days and 8.5% at 28 days after HSCT, respectively. At 58 days after HSCT, analysis of short tandem repeat regions indicated that the recipient cell chimerism was 40.96%. The dose of cyclosporin A was reduced to 50% at 58 days, 25% at 75 days, and 12.5% at 80 days following HSCT. After a reduction of cyclosporin A, donor chimerism over 90% was achieved at 177 days following HSCT, a level of chimerism that has been maintained. The most recent chimerism status test was performed at 31 months after HSCT and showed that 14.37% of cells were recipient cells (85.63% donor). The chimerism status has been sustained without fluctuation. A bone marrow exam performed at 1 year after HSCT showed normocellular marrow with adequate trilineage hematopoiesis. Following HSCT, neutrophil DHRs test have been performed routinely after HSCT. The DHR oxidized peak at 14 days after HSCT was 69.1%, but at 21 days after HSCT, it had increased to 88.3%. From 3 months to 31 months after HSCT, the results of the patient's DHR oxidized peak have stabilized over 90% (Fig. 1).
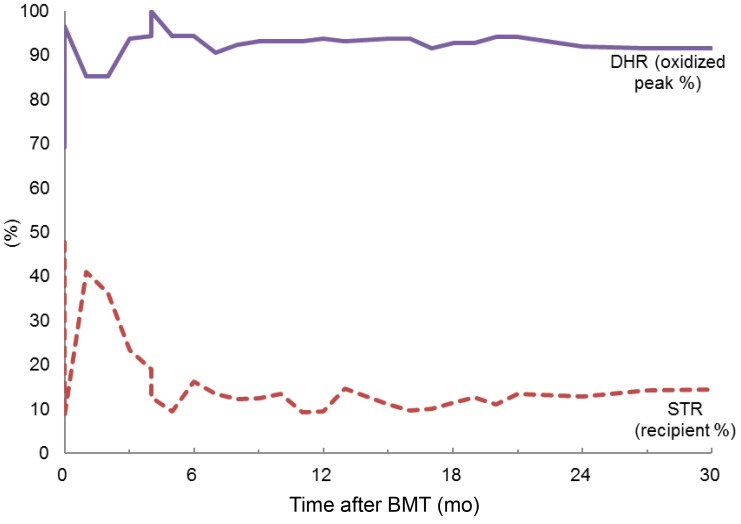
Trends in neutrophil dihydrorhodamine (DHR) test results (oxidized peak %) and short tandem repeat (STR) analysis (% recipient cells) over 31 months following hematopoietic stem cell transplantation. BMT, bone marrow transplantation.
Febrile neutropenia developed 4 days after conditioning but was resolved without severe infection after neutrophil engraftment. No cytomegalovirus or Epstein-Barr virus infections have been detected at routine check-ups. At 45 days after HSCT, the patient presented with symptomatic pericardial effusion which resolved after pericardiocentesis. The pericardial fluid analysis was found to be transudate, and no organisms grew on culture of the fluid. The patient is healthy, and there is no sign of infection or evidence of GVHD, and no relapse of cardiac problem.
This case review was approved by the Institutional Review Board of Seoul National University Hospital (E-1307-037-501).
Discussion
A nonmyeloablative conditioning regimen for CGD showed high engraftment failure rate in a previous study3). Recently, reduced-toxicity myeloablative regimen, including fludarabine with myeloabalative dose of busulfan showed promising results in adult HSCT4). Fludarabine has immunosuppressive property that allows the engraftment of hematopoietic stem cells with minimal extramedullary toxicity, which allows myeloablative conditioning with less toxicity. In this study, we implemented a reduced-toxicity myeloablative conditioning regimen with targeted intravenous busulfan and fludarabine.
Busulfan levels in children are known to vary with age, disease status, and obesity5). Moreover, intraindividual variability has been a problem with the oral form of busulfan, and busulfan AUC variability has been suggested to be related with veno-occlusive disease (VOD) and hepatic toxicity6). After the development of intravenous busulfan formulation, it is used more widely than oral busulfan for the control of pharmacokinetic variability. Busulfan TDM and dose adjustment could be helpful for lowering the toxicity and increasing the efficacy of HSCT7).
In the case presented here, the busulfan AUC level was higher than expected for the first 3 days, and the busulfan dose was adjusted daily according to the actual AUC. On the fourth day, the busulfan AUC was targeted to fit the total sum of AUC for 4 days to the target total AUC. Consequently, the variability of daily AUC was high, but the total AUC of the 4 days approached the target AUC. Because hepatic toxicity and VOD are associated with high total AUC level, a total target AUC <77,000 µg·hr/L was recommended to reduce the toxicity and improve the outcome of HSCT1). There was no definite HSCT-related toxicity related observed in our patient, and this may have been be assisted by busulfan dose adjustment based on TDM.
There have been reports of HSCT in CGD patients who showed survival rates of up to 90%–95%, but engraftment failure has been consistently reported up to 20%. Therefore, reduced-toxicity myeloablative conditioning is preferred by centers to overcome transplantation-related mortality and graft rejection. A recent single center study from the United States of America reported an overall survival of 100% in 11 CGD patients who had undergone HSCT8). Busulfan was given over 4 days with a target total busulfan AUC of 59,114–78,816 µg·hr/L and was combined with cyclophosphamide and cytarabine or cyclophosphamide and fludarabine. The report suggests the prospect of reduced-toxicity myeloablative conditioning with targeted busulfan. Although there have been reports showing poor outcome of HSCT after severe infections9), reduced-toxicity myeloablative conditioning with targeted busulfan, as in our study, could be suggested for CGD patients who previously had severe infection.
A stable mixed chimerism has been maintained in this case. In nonmalignant diseases, long-term stable mixed chimerism was shown to be related to lower rate of bloodstream infection and lower acute GVHD incidence10). The prognosis of mixed chimerism was not worse than full donor chimerism. Hence, mixed chimerism after allogeneic transplant for nonmalignant disease may be observed without further treatment.
This case showed that HSCT based on reduced toxicity myeloablative conditioning regimen containing targeted busulfan, fludarabine can be successfully applied to CGD patients. Based on the result of this case, a prospective phase 1/2 study of HSCT for CGD is in progress, which is registered at www.clinicaltrials.gov (NCT01338675).
Acknowledgments
This research was supported by a grant (11172MFDS288) from Ministry of Food and Drug safety in 2011, and Kyowa Hakko Kirin Korea (6020113640).
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.