Pediatric kidney transplantation is different from adult kidney transplantation
Article information
Abstract
Kidney transplantation (KT) is the gold standard for renal replacement therapy in pediatric patients with end-stage renal disease. Recently, it has been observed that the outcome of pediatric KT is nearly identical to that in adults owing to the development and application of a variety of immunosuppressants and newer surgical techniques. However, owing to several differences in characteristics between children and adults, pediatric KT requires that additional information be learned and is associated with added concerns. These differences include post-KT complications, donor-recipient size mismatch, problems related to growth, and nonadherence to therapy, among others. This review was aimed at elucidating the clinical characteristics of pediatric KT that differ from those observed in adults.
Introduction
Kidney transplantation (KT) was first introduced in 1954, and has become the gold standard for renal replacement therapy in adults with end-stage renal disease (ESRD) owing to research that has provided a better understanding of the human immune system, the remarkable development and application of immunosuppressants, and rapid advancements in surgical techniques. During the initial introductory period, pediatric KT was associated with various technical and immunological difficulties, thereby yielding lower graft- and patient-survival rates in comparison to those observed among adults [1]. Fortunately, over the last 2 decades, remarkable developments have occurred not only in terms of improved patient and graft survival, but also with respect to the improved ability to provide complete rehabilitation and treat comorbidities in this class of patients [2]. It has been reported that at present, the 1- and 5-year patient survival rates associated with pediatric KT are approximately 98% and 94%, respectively, and the 1- and 5-year graft survival rates are 93%–95% (from living donors) and 77%–85% (from deceased donors), respectively [3] (Table 1). An Iranian study has reported that the 5-year graft-survival rate in pediatric recipients who received the first graft from a living donor was lower, perhaps secondary to unreliable adherence to medication regimens, adverse effects of medications, and a higher rate of recurrent disease in this patient population, with no difference observed in terms of patient survival [4]. However, another study has reported a higher rate of acute graft rejections, but a longer patient-survival rate in the pediatric population [5]. According to the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) database, approximately 80% of transplants are performed in pediatric recipients aged >6 years, and approximately 25% of patients undergoing primary transplants undergo preemptive transplantation. Therefore, teenagers represent the largest group of pediatric KT recipients [3]. Although a few characteristics of the clinical KT process are similar in pediatric and adult patients, clinical KT differs between pediatric patients and adults in several aspects, including the causes of ESRD, types of complications, optimal donor selection, problems associated with growth, comorbidities associated with the lower urinary tract, nonadherence to medication regimens, and the child’s transition to adulthood, among others [1].
Causes of ESRD
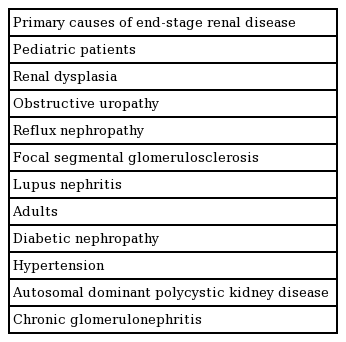
Although ESRD in adults is usually caused by diabetic nephropathy, hypertension, autosomal dominant polycystic kidney, and chronic glomerulonephritis, the primary causes of ESRD in pediatric patients are focal segmental glomerulosclerosis, renal dysplasia, obstructive uropathy, and reflux nephropathy (Table 2). Urological disorders related to anomalies of the lower urinary tract are a significant difficulty encountered in pediatric KT. Treatment of urological disorders may necessitate additional procedures such as open vesicostomy and bladder augmentation. Additionally, recurrent glomerulonephritis after KT, particularly recurrent focal segmental glomerulosclerosis, is a known complication that determines the long-term outcomes of transplantation and is more common in pediatric KT [1,3].
Cardiovascular complications
The risk of cardiovascular complications is one of the most important complications following pediatric KT [2], and cardiovascular mortality is 100 fold higher in pediatric KT than that in an age-matched pediatric population [6]. Cardiovascular disease accounts for a mortality rate of 36% among all pediatric patients with ESRD, 34% among those undergoing dialysis, and 11% of all pediatric deaths after transplantation. Various metabolic conditions that develop during dialysis such as obesity, hyperglycemia, hypercholesterolemia, and hypertension tend to persist even after transplantation; thus, the risk of cardiovascular complications rarely disappears, although it substantially decreases. Additionally, donor-recipient size mismatch is a known factor that increases the pathological cardiac burden in pediatric KT. Among the population of donors, pediatric donors are few in number and are not always suitable for pediatric recipients because of the technical challenges of anastomosis due to small-sized vessels and the risk of thrombosis of the anastomosed area. Therefore, KT in pediatric patients usually involves adult donors, and donor-recipient size mismatch is a commonly encountered difficulty in pediatric KT, particularly in infants and small children. Donor-recipient size mismatch commonly results in graft hypoperfusion and delayed graft function (DGF), which is further complicated by the significantly lower resting blood pressure maintained in small children [7]. Administration of large quantities of intravenous fluids or transfusion, as well as the concomitant use of inotropes may be required to manage hypoperfusion caused by donor-recipient size mismatch. However, management of this fluid status can aggravate the burden on the cardiovascular system.
Posttransplant lymphoproliferative disorder and malignancy
Posttransplant lymphoproliferative disorder (PTLD) is an abnormal proliferation of lymphocytes observed in immunocompromised patients receiving transplantation. Histopathological findings range from an infectious mononucleosis-like presentation to that of non-Hodgkin lymphoma. The risk factors of PTLD include an Epstein-Barr virus (EBV)-seronegative status of recipients, use of calcineurin inhibitors (CNIs) and antilymphocyte antibodies, the number of methylprednisolone pulses administered, cytomegalovirus infection, younger age, and acute graft rejection episodes [8]. Reportedly, the incidence of PTLD is 1% in adults with KT and is higher in pediatric patients, secondary to their more commonly observed EBV-seronegativity status (49% vs. 8%, respectively) [8] (Fig. 1). Additionally, the NAPRTCS transplant registry has shown that these pediatric patients had a 6.7 fold higher risk of non-PTLD malignancies compared to a healthy pediatric population. Renal cell carcinoma was the most common type of non-PTLD malignancy observed [9].
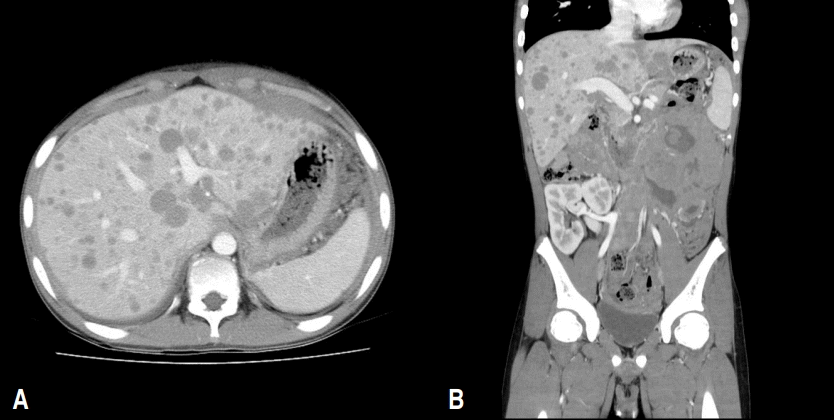
Abdominal computed tomography (CT) scan obtained from a 17-year-old male adolescent who received a renal transplant. This patient was diagnosed with end-stage renal disease secondary to focal segmental glomerulosclerosis 6 years prior to presentation and received a renal transplant from his mother. (A, B) The CT scan shows a large heterogeneous mass in the left upper quadrant of the abdomen, diffuse peritoneal thickening, and multiple liver metastases, which are findings consistent with posttransplant lymphoproliferative disorder.
Donor-recipient size mismatch
Much research has been performed to determine the ideal kidney donors for pediatric recipients. In terms of the suitability of a deceased donor kidney, the following factors must be considered in pediatric KT recipients awaiting a kidney transplant from a deceased donor: renal function of the donor kidney, age of the donor, donor criteria, and matching degree of human leukocyte antigen (HLA). Elevated serum creatinine levels in donors can predispose to DGF and poorer long-term prognosis. Kidneys obtained from very young donors can be associated with graft thrombosis owing to small-sized anastomotic vessels. Kidneys from expanded criteria donors and those diagnosed with cardiac death are inappropriate owing to the possibility of DGF and poorer long-term graft survival. Moreover, most pediatric recipients require retransplantation; therefore, kidneys from HLA-mismatched donors are not preferred [10].
Unfortunately, given the current scarcity of available organ donors, longer waiting times are inevitable to meet these criteria for an optimal kidney donor for pediatric recipients [7].
In the United States, however, the United Network for Organ Sharing initiated an allocation policy in 2005 called “Share 35,” which preferentially allocated kidneys from deceased donors aged <35 years to pediatric recipients aged <18 years. The purpose was to reduce the waiting time in pediatric KT patients and increase the availability of high-quality donors for such patients [7].
The surgical technique utilized for an adult KT is similar to that used in pediatric patients with a body weight >30 kg. However, a midline longitudinal abdominal incision is necessary in a pediatric patient with a body weight <10 kg. Since the space between the peritoneum and the subcutaneous fascia is restricted, the kidney needs to be placed intraperitoneally, with the attendant risk that it may move to another part of the peritoneal cavity. The renal vein and artery of a graft are anastomosed to the recipient’s inferior vena cava and aorta. In pediatric patients with a body weight of 10–30 kg, surgeons individualize the incision and allograft sites based on the child’s anatomy [1]. A few transplant surgeons choose an extraperitoneal (retroperitoneal) surgical approach for KT, even in small children, owing to the lower risk of bowel complications, possibility of peritoneal dialysis after KT, and easier access to transplant biopsy that are associated with this aforementioned approach [11]. Vitola et al. [12] reported a study comprising 62 patients in whom an extraperitoneal approach was used for surgical access to perform KT in pediatric recipients weighing <15 kg. They faund the extraperitoneal approach to be practical for KT in that pediatric patient population.
Immunosuppression
Presently, several pediatric transplantation centers utilize immunosuppression protocols similar to those used in adults. However, steroid avoidance is more important in pediatric patients than in adults. Although steroid therapy has been a keystone of most immunosuppressant regimens utilized in KT, steroid use is associated with various well-known adverse effects including growth retardation, hypertension, glucose intolerance, hyperlipidemia, cataract formation, diabetes, fracture, osteoporosis, and mood and cosmetic changes [2]. Therefore, several different strategies to minimize steroid doses have been attempted in pediatric KT including early and late steroid withdrawal and complete steroid avoidance, among others [13].
Attempts to avoid steroid withdrawal in pediatric KT were first observed in the late 1980s. However, maintenance steroid therapy was essential in pediatric patients owing to the higher risk of acute rejections observed after withdrawal [2]. A retrospective case control study comprising pediatric KT reported that the early discontinuation of steroids led to a lower delta body mass index, lower rate of hyperlipidemia, and a higher height z-score without increasing the risk of rejection [14]. A prospective study performed by a Stanford University group analyzed a complete steroid avoidance protocol and suggested that a complete steroid-free regimen is effective and safe in the low-risk pediatric group without subsequent increase in the risk of early acute rejection [15]. Catch-up growth was most noticeable in the group comprising the youngest patients (those aged <6 years) among pediatric KT recipients who received steroidfree therapy [16]. One randomized controlled trial (RCT) has reported the use of sirolimus, basiliximab, steroids, and CNIs in pediatric KT patients over 6 months. Among the enrolled patients, 45% were randomized to maintain steroid therapy, whereas 55% underwent steroid withdrawal. The steroid-free group showed a higher incidence of acute rejection and a statistically significant incidence of graft loss or death at the 3-year follow-up (P<0.002) [17]. From this multicenter RCT, it was concluded that the 3-year follow-up of a steroid-free protocol with daclizumab in low-risk recipients at initial transplantation was effective in comparison to pediatric patients treated with steroids and did not increase the incidence of PTLD [18]. Based on a recent systematic review, the role of a steroid-withdrawal regimen remains disputable. An analysis of 9 RCTs has revealed comparable mortality and graft failure rates between the steroid withdrawal and control groups. However, the prevalence of other complications differed based on the type of CNI used (cyclosporine vs. tacrolimus) [2]. For example, the cyclosporine trial showed that steroid avoidance was associated with a greater number of episodes of acute rejection but a lower rate of the common complication of new-onset diabetes mellitus, whereas the tacrolimus trial showed no difference in the prevalence of new-onset diabetes mellitus and acute rejection based on steroid avoidance. The conclusions drawn from this meta-analysis were that a steroid-withdrawal regimen is safe and effective in KT recipients receiving induction treatment with thymoglobulin or anti-interleukin-2 receptor antibodies, although the long-term effect of these regimens remains unclear.
Growth
Post-KT complications include left ventricular hypertrophy and several complications of metabolic bone disease including skeletal deformities, bone pain, fracture, osteonecrosis, growth failure, and ectopic calcification [19,20]. Risk factors associated with posttransplant growth retardation in pediatric patients include older age at transplantation (>6 years), decreased allograft function, and higher corticosteroid dosage [21]. Recipients of living-related donor grafts are taller at all ages and show superior growth velocity during infancy and puberty than that in recipients of cadaveric donor grafts [22].
Strategies to improve linear growth after pediatric KT include transplantation at a younger age (<6 years), use of steroid-avoidance or withdrawal regimens, and use of recombinant human growth hormone (rhGH). Glucocorticoids interfere with the width of the growth plate, increase the apoptosis of chondrocytes, and reduce vascular endothelial growth factor expression as demonstrated by in vivo studies. Reportedly, the use of rhGH is associated with an increased incidence of renal graft cell carcinoma and acute rejection in patients with a history of acute rejection. However, based on the NAPRTCS transplant registry, it has been reported that treatment with rhGH causes a significant increase in height and enables the final adult height to be reached without a decline in graft function. Therefore, by preventing an acute rejection episode for a minimum duration of 12 months, rhGH offers significant advantages in this context [23].
Nonadherence & transition into adulthood
Nonadherence to medical recommendations is widespread, with rates as high as 75% among adolescents, and this is an important factor that causes unfavorable outcomes following otherwise successful pediatric KTs. In general, a child’s transition into adulthood is a critically vulnerable period [24,25]. Primary risk factors of nonadherence include poor family functioning and poor psychological functioning of the child. Poor family functioning includes poor family cohesion and dysfunctional family dynamics [26]. In a recent review [27], Rianthavorn and Ettenger expressed their disappointment in this regard: “The long-term transplant outcome in adolescents is disappointing in spite of the best 1-year graft survival. Nonadherence with immunosuppressive medications is one of the most significant contributing factors for graft rejection and loss in adolescents.” Therefore, nonadherence should be monitored using objective methods such as pill counts, medication refill rates, blood levels of medications, and thorough use of electronic devices. Effective health education that includes imparting behavioral skills and using motivational strategies is warranted to assist such adolescents [24,25].
Successful transition of care requires that age-appropriate practices be adopted by patients undergoing transplantation, their parents, and the staff involved with performing pediatric and adult transplantations. For example, pediatric transplant patients should be instructed as to their medical condition, its treatments, the need for treatment during childhood and adolescence, and optimal selfcare practices, and they should understand and accept the eventual need for transfer of care. Although parents should be responsible for the medical care of their children, patient autonomy is important. Staff involved with pediatric transplantation should be equipped to discuss issues regarding sexuality, ensuring confidentiality, and must transfer their pediatric patients to adult practitioners when the children no longer belong in the pediatric age group [28].
Conclusions
Clinical characteristics of pediatric KT differ from those of adults in terms of several aspects including the causes of ESRD, types of complications, optimal donor selection, growth issues, comorbidities associated with the lower urinary tract, nonadherence to medications, and transition into adulthood. Therefore, successful pediatric KT requires a multidisciplinary approach with effective interagency coordination between pediatric nephrologists, urologists, transplantation surgeons, social workers, pharmacists, and clinical coordinators, with pediatric nephrologists positioned at the center of this team.
Notes
No potential conflict of interest relevant to this article was reported.