Autoimmunity and intestinal colonization by Candida albicans in patients with type 1 diabetes at the time of the diagnosis
Article information
Abstract
Purpose
Type 1 diabetes mellitus (T1DM) is a chronic and immune-mediated disease, which is characterized by the progressive destruction of pancreatic beta cells. T1DM precipitates in genetically susceptible individuals through environmental factors. In this study, we aimed to evaluate the impact of autoimmunity and intestinal colonization of Candida albicans on the development of T1DM.
Methods
Forty-two patients newly diagnosed with T1DM and 42 healthy subjects were included in this monocentric study. The basic and clinical characteristics of the patients were recorded. T1DM-, thyroid-, and celiac-associated antibodies were evaluated. Stool cultures for C. albicans were performed to assess whether or not gut integrity was impaired in patients with T1DM.
Results
The evaluation of T1DM- and thyroid-associated antibodies showed that the prevalences of islet cell antibodies and antithyroperoxidase positivity were higher in the study patients than in the patients in the control group. Furthermore, the direct examination and culture of fresh stool samples revealed that 50% of the patients with T1DM and 23.8% of the control subjects had fungi (C. albicans).
Conclusion
Through this study, we suggest that the presence of intestinal C. albicans colonization at the time of the diagnosis of T1DM may indicate impairment of normal intestinal microbiota. We also suggest that there may be a tendency of T1DM in patients with a high prevalence of intestinal C. albicans.
Introduction
Diabetes is a group of chronic diseases characterized by hyperglycemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action, or both [1]. Type 1 diabetes mellitus (T1DM) is known to be the result of the selective damage of pancreatic cells. The destruction of the cells is the consequence of a cell-mediated immune response mediated by islet-infiltrating lymphocytes and macrophages (insulitis) [2]. It has been estimated that more than 300 million people worldwide are affected by this autoimmune condition and the rate of incidence of T1DM onset is increasing about 3% yearly especially in young children with marked geographical differences among the different countries [3-5]. Due to a recent data about pediatric T1DM prevalence in a large pediatric population in Istanbul, Turkey, estimated prevalence of T1DM has been reported as 0.67/1,000 [6].
T1DM is a chronic autoimmune disorder that precipitates in genetically susceptible individuals by environmental factors [7]. T1DM is often associated with other autoimmune diseases such as autoimmune thyroid disease, celiac disease (CD), autoimmune gastritis, pernicious anemia and vitiligo. Emerging evidence shows that intestinal microbial environment could be involved in the pathogenesis of many forms of autoimmune and metabolic diseases, including T1DM [8]. The nexus of intestinal microbiota composition, the intestinal barrier, and the mucosal immune system have been investigated for their potential role for development of T1DM. Recent animal studies revealed that changes in the normal flora could be of importance in the development of autoimmune diabetes by affecting intestinal permeability [9,10]. In this study, we aimed to evaluate the impact of impaired intestinal microbiota and gut colonization by Candida albicans in the development of T1DM.
Materials and methods
Forty-two newly diagnosed T1DM patients and 42 healthy subjects, who were admitted to Istanbul Kanuni Sultan Suleyman Training and Research Hospital between September 2010 and February 2011, were included in this prospective monocentric study. The patients with previously diagnosed T1DM patients and type 2 diabetes patients were not included in the study. Patient characteristics including gender, age, weight, height, birth weight, type of delivery, postnatal history, duration of hospitalization, and past medical history were recorded. The symptoms on admission and clinical findings were noted. The nutritional status during the first 6 months (breast feeding, use of rice cereal, starch, etc.) were also obtained. Furthermore the time of introduction of cow-milk feeding was recorded.
In this study, several autoantibodies for subjects were evaluated; type 1 diabetes-asoociated autoantibodies (insulin autoantibody [IAA], islet cell antibody [ICA], anti-glutamic acid decarboxylase [anti-GAD] antibodies), thyroid autoantibodies (anti-thyroglobulin [anti-TG] and microsomal antigens [TPO]), celiac antibodies (antigliadin antibodies of IgA class, antigliadin antibodies of IgG class, and antiendomysium antibodies). In addition, glycosylated hemoglobin, 25-hydroxy vitamin D3 levels were evaluated. Stool cultures for C. albicans were studied in Sabouraud Dextroz Agar to assess whether gut integrity was impaired in T1DM patients or not.
Continuous variables were presented as medians and interquartile ranges or as mean±standard deviation. Categorical variables were presented as observed frequencies and percentages. Continuous variables were compared using Student t test or the Mann-Whitney U test as appropriate. Categorical variables were compared using the chi-square test. The statistical analyses were performed using the SPSS ver. 17 (SPSS Inc., Chicago, IL, USA). P values of <0.05 were considered significant. All participants provided written informed consent for participation in the study, which was approved by the institutional ethics committee (approval number: 2010/3).
Results
The demographic characteristics of 42 T1DM patients and 42 healthy subjects are summarized in Table 1. The majority of the study patients were between 4 and 9 years-old. The most frequent symptoms on admission were polyuria, polydipsia, weight loss and fatigue. Ketoacidosis was present in 19 patients (45.2%).
Rice-cereal-based feeding in the first 6 months was more frequent in the control group compared to patients with T1DM (P=0.026) whereas there was no statistical significance between the groups in terms of feeding with other type of nutritions (P>0.05). On the other hand, history of cow milk-based feeding in the first year of life was statistically more frequent in the patients with T1DM compared to controls (P=0.038).
When 2 groups were compared in terms of ICA, IAA and anti-GAD antibodies, prevalence of ICA positivity was found to be higher in the patients with T1DM compared to controls (P<0.01), whereas IAA and anti-GAD positivity were similar between the 2 groups (P>0.05). The presence of anti-TG and anti-TPO antibodies was showed in only T1DM patients group (6 T1DM subjects had anti-TPO and 1 T1DM subject had anti-TG antibodies). CD was detected in 2 patients shortly after initial diagnosis of T1DM on the basis of clinical features and positive test results for CD. The results of stool culture and diabetes, thyroid and celiac related autoantibodies in 2 groups are presented in Fig. 1.
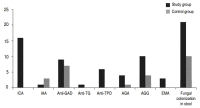
The findings of stool culture and diabetes-, thyroid-, and celiacassociated autoantibody tests in the 2 groups. ICA, islet cell antibody; IAA, insulin autoantibody; GAD, glutamic acid decarboxylase; TG, thyroglobulin; TPO, microsomal antigens; AGA, antigliadin antibodies of IgA class; AGG, antigliadin antibodies of IgG class; EMA, antiendomysium antibodies.
In diabetic and control groups, vitamin D3 levels were 42.98± 30.44 ng/mL and 34.74±13.5 ng/mL, respectively (P>0.05). The direct examination and culture of the fresh stool sample revealed fungi (C. albicans) in half of the diabetic patients and in 23.8% of the control subjects (P<0.01).
Discussion
Genetic, environmental and autoimmune factors play essential role in the onset of T1DM which is a common endocrine disorder of childhood and adolescence. In this study, we showed that impairment of intestinal microbiota by C. albicans could be associated with autoimmune diabetes-T1DM. Beta-cell autoimmunity also plays an important role in the pathogenesis of T1DM and the presence of ICA is of particular value for prediction of the disease. Besides, the presence of both IAA and ICA are associated with greater risk for DM [11,12]. In present study, no significant statistical difference was found between diabetic and control subjects in terms of IAA and GADA, whereas ICA was significantly higher in diabetic group.
In several publications, it has been reported that thyroid autoimmunity is 2–4 times more common in patients with T1DM compared to normal population. The frequency of thyroid autoantibodies varies between 6.6% and 10% in healthy population, but this rate can be as high as 20% to 40% in T1DM. It has been suggested that thyroid autoantibodies usually become positive after 3–3.5 years of initial T1DM diagnosis [13]. In our study, anti-TG antibodies were detected in 1 and anti-TPO antibodies in 6 patients at the time of diagnosis of T1DM. Also, one of our patients had already been treated for autoimmune thyroiditis before the initial diagnosis of T1DM. Anti-TPO positivity was significantly higher in the diabetic group when compared with the control group; however, thyroid function tests were normal in this study group.
According to current data, the prevalence of CD is 5–7 times higher in diabetic patients compared to general population. The percentage of diabetic children with histopathologically confirmed CD varies from 2.4% in Finland to 16.4% in Algeria [14]. In T1DM patients with CD, typical gastrointestinal findings such as diarrhea and abdominal distention are rarely seen, whether atypical findings such as sideropenic anemia, short stature or delayed puberty are more commonly observed [15]. However, CD may be clinically silent and the patients may be asymptomatic; in this circumstance only the serological markers can be identified [15]. In the present study, CD was diagnosed in 2 patients shortly after the initial diagnosis of T1DM.
It is becoming increasingly clear that the nexus of intestinal microbiota composition, the intestinal barrier, and the mucosal immune system plays a pivotal role in the development of various allergic and autoimmune diseases [8]. In patients with T1DM, increased intestinal permeability, the signs of enhanced immune activation documented by intestinal biopsy and ultrastructural changes have been shown [16-19]. Bosi et al. [20], hypothesized that if the integrity of the intestinal epithelium is primarily compromised by any injury (toxic, infectious, inflammatory, etc.), an autoimmune reaction could develop as a response to abnormally absorbed macromolecules. Conversely if an immune-mediated process is primarily taking place at the intestinal level regardless of the trigger, mucosal integrity might be compromised due to associated inflammatory process. They stated that presence of a subclinical enteropathy could be associated with the autoimmune process as an underlying cause of T1DM and the small intestine is important in the pathogenesis of T1DM [20]. However, intestinal colonization by C. albicans can cause diarrhea, fatigue, nutritional intolerance, arthritis, and skin problems. Increased gut permeability secondary to mucosal inflammation may lead to future autoimmune and allergic diseases. In 2011, Sonoyama et al. [21] reported that eosinophil and mast cell infiltration is higher in Candida colonized mice than in those without Candida colonization. In another study, it has been shown that Th2 cells stimulate mast cells and eosinophils in the large intestine of diarrhea-induced mice and Th2-type cytokines play a critical role in the development of allergic responses [22]. In a recent animal experiment, fecal samples taken from diabetic sensitive and resistant rats were compared and the authors determined that the ratio of Bifidobacter and Lactobacilli was much higher in the diabetic resistant group compared with the diabetic sensitive group [23]. This suggests that the normal intestinal flora changes over time in autoimmune diseases such as T1DM. In our study, the positive stool culture for C. albicans was found in half of the diabetic group and in almost 25% of the control group, which was statistically significant. As C. albicans positivity was detected at the time of diagnosis, we can argue that increases in intestinal permeability due to deterioration of the normal intestinal microbiota might have triggered the development of autoimmune processes (such as T1DM).
The newborns delivered by cesarean section have a reduced number of bacteria compared with those vaginal delivered infants and the appearance of Bifidobacteria seems to be delayed for up to 6 months of life [24]. In full term and breastfed infants, gram-positive Bifidobacteria, Lactobacilli, and streptococci are dominate in the intestinal flora whereas the predominate flora in formula-fed infants is a mixture of Enterobacteriaceae, Staphylococcus, Clostridium, Bifidobacterium, Enterococcus, and Bacteroides species [25]. In our study group, 15 patients were born by cesarean section. A quarter of the patients were fed with a combination of breastmilk and other nutrients including fecula and foods with rice. Cow-milk feeding within the first year of life was detected in 15 patients (35%). When the diabetic group is assessed with regard to type of birth and nutrition; it can be determined that the intestinal microbiota of the diabetic group did not include the microorganisms that are required for the development of a normal immune system. This situation may lead to development of autoimmune diseases. As a result, we should reach the general population, explain the importance of breastfeeding in the first 6 months and make the public more conscious about this issue.
There is a link between the gut microbial composition and various aspects of human health. Also, there are some studies which have described the physiopathology of T1DM based on disturbance of the intestinal flora [26,27]. The presence of intestinal colonization with C. albicans at the time of the diagnosis, which is an opportunistic fungus, may indicate the impairment of normal intestinal bacterial microbiota. These changes in the intestinal microbiota and inaccuracies in infant feeding (cows’ milk, other nutrients including fecula and foods with rice in the early infantile period) may promote the development of many autoimmune disorders. We suggest that there may be a tendency to T1DM in patients with high prevalence of intestinal C. albicans. Understanding the underlying mechanisms of the etiology of T1DM is critical to solve the complex pathogenesis of the disease and to gain new perspectives for future approaches.
The sample size of subjects was relatively small and intestinal microbiota analysis could not be done in our study. In addition, the infants who were fed without rise-based foods and other nutrients in the infantile period, could be selected as the control group.
Notes
No potential conflict of interest relevant to this article was reported.

