Serum alanine aminotransferase levels are closely associated with metabolic disturbances in apparently healthy young adolescents independent of obesity
Article information
Abstract
Purpose
Liver metabolism plays a pivotal role in the development of metabolic disorders. We aimed to investigate the clinical and laboratory risk factors associated with alanine aminotransferase (ALT) levels in young adolescents from an urban population in Korea.
Methods
A population of 120 apparently healthy adolescents aged 12–13 years was included in the cross-sectional design study; 58 were overweight or obese and 62 were of normal weight. We estimated anthropometric and laboratory measurements, including waist-to-height ratio, blood pressure, insulin sensitivity, aspartate aminotransferases (AST), ALT, and lipid profiles.
Results
The mean ages of the overweight or obese and normal weight participants were 12.9±0.3 and 13.0±0.3 years, respectively. Height, weight, body mass index, waist circumference, waist-to-height ratio, systolic and diastolic blood pressure, AST, ALT, total cholesterol, low-density lipoprotein-cholesterol, triglyceride, insulin, and the homeostatic model assessment of insulin resistance (HOMA-IR) score were significantly higher and the high-density lipoprotein-cholesterol and quantitative insulin-sensitivity check index were significantly lower in the overweight/obese participants in comparison to the normal-weight participants (all P<0.05). In multivariate linear regression analysis, waist-to-height ratio, systolic blood pressure, and HOMA-IR score were independently and positively associated with serum ALT levels.
Conclusion
Screening for ALT levels in adolescents may help to differentiate those at risk of metabolic abnormalities and thus prevent disease progression at an early age.
Introduction
Obesity has emerged as a large public health concern and is now developing at younger ages around the world [1]. Mounting evidence reports that childhood obesity frequently continues into adulthood and causes the risk of various diseases, such as cardiovascular diseases, diabetes mellitus, and nonalcoholic fatty liver disease (NAFLD), ultimately leading to increased morbidity and mortality [2].
NAFLD is at present the most common cause of liver diseases among children and adolescents [3,4]. With the surging epidemic of childhood and adolescent obesity, NAFLD is recognized as a major health threat in young generations. Previous studies have indicated that NAFLD is the hepatic manifestation of overweight [5], obesity [6], and metabolic syndrome [6,7]. Although several studies [8-10] have investigated the risk of NAFLD among particular pediatric and adolescent groups, there have been scanty studies determining the risk factors associated with this disorder. The lack of data hinders our ability to establish effective and appropriate primary prevention programs and services for young generations at risk of developing liver diseases and obesity-associated disorders. If undiagnosed and untreated, NAFLD in children and adolescents may progress silently and ultimately lead to liver cirrhosis, portal hypertension, and liver-related death in early adulthood [11]. In many countries, liver cirrhosis and other liver diseases are one of the main causes of general mortality and death among adults [12-14]. Determining ways to differentiate subjects at risk of developing NAFLD may be critical to help reduce the burden of liver disease in adolescents.
The current research aimed to determine whether there are differences in serum alanine aminotransferase (ALT) levels between overweight/obese subjects and normal-weight subjects and to identify risk factors associated with ALT concentrations among apparently healthy young adolescents.
Materials and methods
1. Study subjects
One hundred and fifty-one adolescents aged 12–13 years performed Student Health Examinations at a local health check-up clinic in Seoul, Korea between June and August 2015. Subjects who met the following exclusion criteria were not included in the analysis (n=31): any missing covariate data; a medication history of steroids, insulin, glucose regulators, or antihypertension medications; participants who refused the anthropometric measurements or blood test; participants who had signs and/or symptoms of acute infections; or participants who had not fasted for a minimum of 12 hours before blood test. After these exclusions, 120 adolescents (69 boys and 51 girls) were included in the final investigation. Health check-ups were conducted by a single pediatrician in accordance with a systematized process. Body weight and height were quantified to the nearest 0.1 kg and 0.1 cm, respectively, with an automatic height–weight scale while the study participants wore light indoor clothing and no shoes. Body mass index (BMI) was computed as weight (kg)/height (m2). The BMI percentiles for age and sex were calculated according to the 2017 Korea Growth Charts [15]. The participants were categorized into 2 groups according to their BMI (i.e., overweight/obese and normal-weight participants). A certified technician measured blood pressure (BP) a maximum of three times on the right arm with the participants seated after a 5-minute rest using an automatic BP recorder. The development of secondary sexual characteristics was measured in accordance with Tanner stages by the pediatrician. The institutional review board of the CHA Gangnam Medical Center approved the study (IRB No. GCI-115-04) and written informed consent was attained from guardians and consent/assent from the adolescents.
2. Laboratory analyses for obesity-related biomarkers
After a 12-hour overnight fast, blood specimens were attained from the antecubital vein of the participants by venipuncture and were promptly centrifuged, aliquoted, and frozen at -20°C. The frozen serum and plasma samples were accumulated at -80°C until final analysis. Fasting plasma glucose, total cholesterol, triglyceride, and high-density lipoprotein-cholesterol (HDL-C) levels were quantified by enzymatic methods using a Hitachi Modular D2400 automated chemistry analyzer (Hitachi, Tokyo, Japan). Levels of low-density lipoprotein-cholesterol (LDL-C) were determined using the following equation: LDL-C=total cholesterol − HDL-C − (triglyceride/5). Fasting insulin concentrations were assessed using a chemiluminescent microparticle immunoassay (Abbott Architect System, Irving, TX, USA). We also subjected the fasting data to various transformations and ultimately defined quantitative insulin sensitivity check index (QUICKI=1/[log(I0)+log(G0)]), where I0 is the fasting insulin, and G0 is the fasting glucose. Insulin resistance was computed using the homeostatic model assessment of insulin resistance (HOMA-IR) and assessed using the following formula: HOMA-IR=(fasting insulin [μIU/mL]×fasting glucose [mg/dL]/18)/22.5 [16].
3. Serum AST and ALT measurement
Serum aspartate aminotransferases (AST) and ALT levels were measured by the catalytic concentration from the rate of decrease of nicotinamide adenine dinucleotide measured at 340 nm by means of lactate dehydrogenase coupled reaction.
4. Statistical analysis
The normality of continuous variables was evaluated by the Kolmogorov-Smirnov test. All continuous variables are presented as mean±standard deviation, median (interquartile range), or are displayed as scatter plots. Comparisons of group differences for continuous variables were tested by the Mann-Whitney U test or the Student t test. Categorical variables are shown as an absolute number with the corresponding proportion. The significance of differences in proportions was assessed by the chi-square test. Spearman correlation analysis was implemented to determine the association between serum ALT concentrations and anthropometric measurements and obesity-associated biomarkers. The study subjects’ anthropometric and biochemical parameters with regards to ALT quartiles were computed using the analysis of variance for continuous variables and the chi-square test for categorical variables. ALT quartiles were classified separately as follows: Q1, ≤10.0; Q2, 10.0–13.0; Q3, 13.0–20.0; and Q4, ≥20.0. To determine independent correlates of serum ALT concentrations, a multivariate linear regression analysis was utilized with the ALT level as the dependent variable. All statistical analyses were performed using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA). All statistical tests were 2-sided, with a P value of <0.05 indicating statistical significance.
Results
1. Characteristics of the study participants
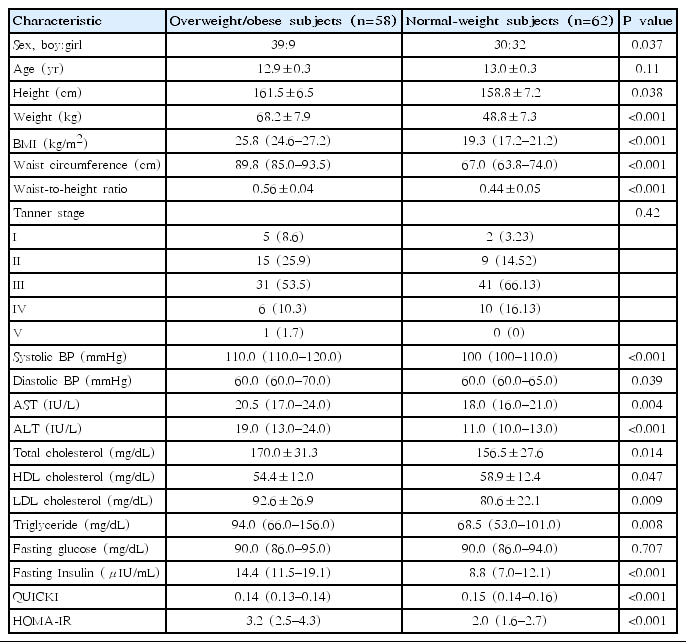
There were 58 subjects with overweight/obesity and 62 with normal-weight, respectively (12.9±0.3 years vs. 13.0±0.3 years, P=0.11) (Table 1). There were more boys in the overweight/obese subjects than in the normal-weight subjects (P=0.037) (Table 1).
2. Comparison of clinical and laboratory parameters between overweight/obese and normal-weight subjects
Table 1 indicates the comparison of the clinical and laboratory parameters for overweight/obese and normal-weight subjects. In the anthropometric measurements, height, weight, BMI, waist circumference, waist-to-height ratio, and systolic and diastolic BP were significantly higher in the overweight/obese subjects in comparison with normal-weight subjects (all P<0.05). There were no significant differences in age or Tanner stages between groups. In the laboratory parameters, AST, ALT, total cholesterol, LDL-cholesterol, triglyceride, fasting insulin, and HOMA-IR were significantly higher and HDL-cholesterol and QUICKI were significantly lower in the overweight/obese subjects when compared with normal-weight subjects. There was no difference in fasting glucose between groups.
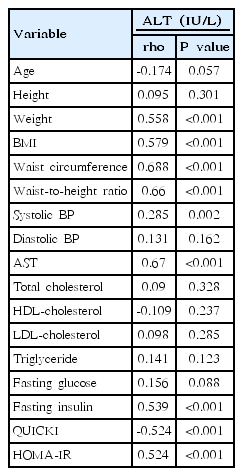
3. Associations of serum ALT levels with anthropometric measurements and obesity-related biomarkers
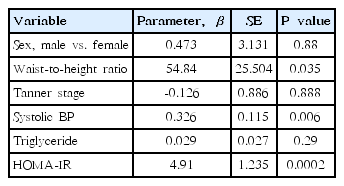
Spearman correlation results between ALT and various parameters are depicted in Table 2. ALT level was significantly and positively correlated with weight (rho=0.558, P<0.001), BMI (rho=0.579, P<0.001), waist circumference (rho=0.688, P<0.001), waist-to-height ratio (rho=0.66, P<0.001) (Fig. 1A), systolic BP (rho=0.285, P= 0.002) (Fig. 1B), AST (rho=0.67, P<0.001), fasting insulin (rho=0.539, P<0.001), QUICKI (rho=-0.524, P<0.001), and HOMA-IR (rho=0.524, P<0.001) (Fig. 1C). No significant correlations were detected between ALT levels and age, height, diastolic BP, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglyceride (rho=0.141, P=0.123) (Fig. 1D), or fasting glucose. Weight, BMI, waist circumference, waist-to-height ratio, systolic BP, AST, ALT, fasting insulin, and HOMA-IR increased and QUICKI decreased significantly in accordance with ALT quartiles (all P<0.05) (Table 3). In a multivariate linear regression analysis, waist-to-height ratio, systolic BP, and HOMA-IR were independently and positively associated with serum ALT levels (Table 4).
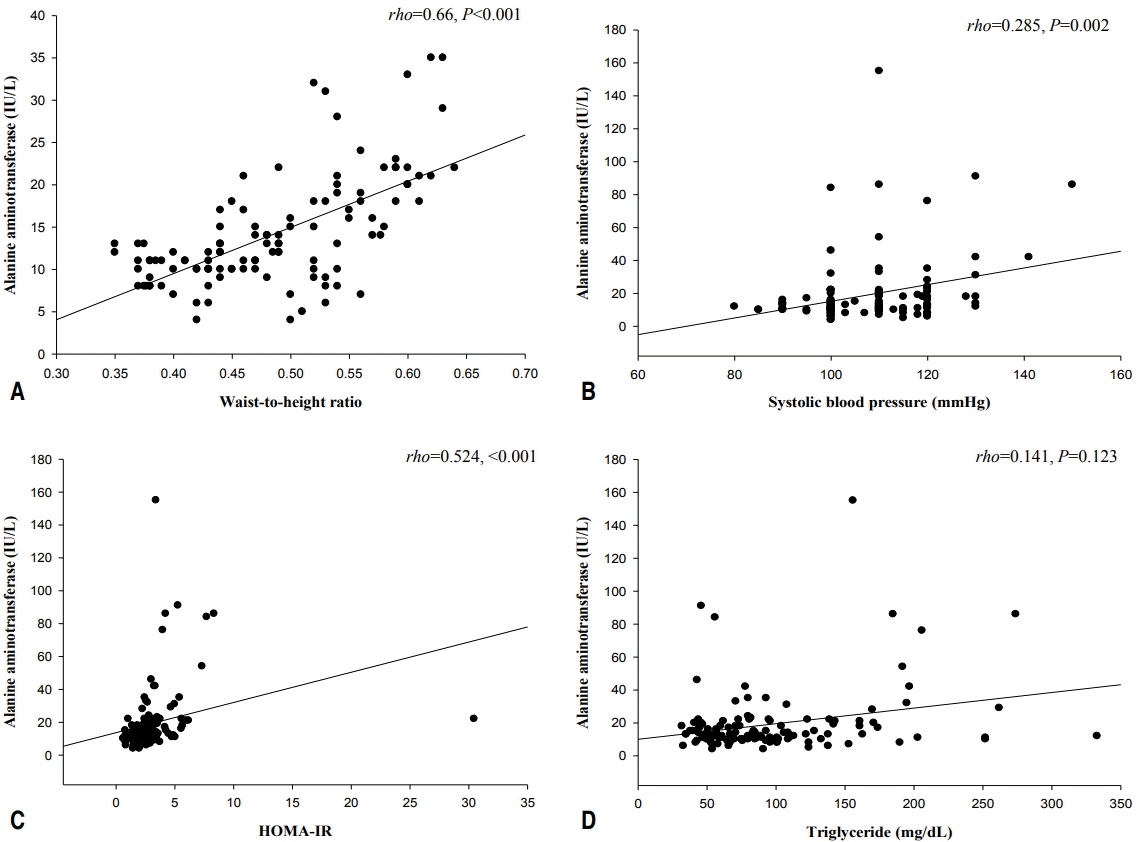
Scatter plots of serum alanine aminotransferase levels vs. waist-to-height ratio, systolic blood pressure, homeostatic model assessment of insulin resistance score, and triglyceride in apparently healthy young adolescents. (A) Alanine aminotransferase vs. waist-to-height ratio; (B) alanine aminotransferase vs. systolic blood pressure; (C) alanine aminotransferase vs. homeostatic model assessment of insulin resistance (HOMA-IR); and (D) alanine aminotransferase vs. triglyceride.

Anthropometric and biochemical parameters according to alanine aminotransferase levels in the study subjects
Discussion
The current study examined whether there are differences in serum ALT concentrations between overweight/obese and normal-weight subjects and to differentiate risk factors associated with ALT concentrations among apparently healthy young adolescents. We found a significant difference in serum ALT levels between overweight/obese and normal-weight subjects and a significant positive correlation between serum ALT levels and waist-to-height ratio, systolic BP, and HOMA-IR in a multivariate linear regression analysis. To the best of our knowledge, this is the first study to determine a significant positive association between ALT concentrations and waist-to-height ratio, systolic BP, and HOMA-IR in a population of apparently healthy young adolescents.
The prevalence of obesity and obesity-related-metabolic disorders are on the rise across the globe [1]. Despite vigorous research effort, the pathogeneses and pathophysiology of these complex diseases remain elusive. In recent times, previous studies have reported that obese children and adolescents have higher serum ALT levels than controls, indicating that ALT may be implicated in the pathogenesis of obesity. A recent representative study of 1,262 participants aged 8–19 years in Mexico has reported that overweight and obese youth have higher serum ALT concentrations than controls and that abdominal obesity and insulin resistance are associated with an increased risk of elevated ALT (>40 U/L) [17]. Another study comprising 5,586 adolescents aged between 12 and 19 years in the U.S. National Health and Nutrition Examination Survey 1999–2004 has reported that ALT level is associated with waist circumference and insulin resistance [18]. Recently, a representative study of 886 subjects with the mean age of 15 years in the 2009–2010 Korea National Health and Nutrition Examination Survey has shown that boys with the highest ALT quartile were more likely to have the highest quartile of insulin resistance [19]. In agreement with previous studies, the present study determined significantly increased serum ALT concentrations in adolescents with overweight/obesity than those of normal-weight. In addition, we found a significant positive correlation between ALT and waist-to-height ratio, systolic BP, and HOMA-IR, even after adjustment for potential confounders. Taken together, these findings indicate that measurement of serum ALT concentrations may provide clinicians with a useful biochemical marker for apparently healthy young adolescents at risk of metabolic deregulations.
Emerging evidence has indicated that the liver metabolism is implicated in the pathogenesis of metabolic syndrome [20] and insulin resistance [18,20]. NAFLD is the accumulation of large droplets of triglycerides in liver cells without a history of chronic alcohol consumption and, therefore, has been recognized as the hepatic manifestation of overweight [5], obesity [6], and metabolic syndrome [6,7]. Despite the fact that liver biopsy is the gold standard for establishing NAFLD, serum biomarkers, particularly ALT levels, have been demonstrated to be highly precise in diagnosing NAFLD. These biomarkers are also well correlated with risk of overweight, obesity, metabolic syndrome, diabetes mellitus, and cardiovascular disease [7,21,22]. Furthermore, previous research have demonstrated that the liver enzymes levels rise prior to the development of nonalcoholic fatty liver, proposing that liver enzymes may be a surrogate marker of NAFLD [6,7,20,23]. Although we did not conduct a liver biopsy study, we found that the serum ALT levels were significantly and positively associated with abdominal obesity estimated using the waist to-height ratio and certain metabolic risk factors including BP and insulin resistance estimated using the HOMA-IR in a group of apparently healthy young adolescents in the multivariate linear regression analysis.
There may be congruent metabolic mechanisms to consider as to why serum ALT is positively associated with waist-to-height ratio and certain metabolic risk factors. One possible underlying mechanism for the current finding is as follows. ALT is a liver enzyme that is released in serum as a consequence of hepatocyte damage. If the aggregation of body fat becomes overloaded, adipose tissue releases free fatty acid into the hepatic portal vein; consequently, this restricts the physiological measure of eradicating insulin resistance. Insulin resistance is known to increase uptake of free fatty acid into liver, and the absorbed hepatic fatty acid is resynthesized into triglyceride, ultimately leading to the development of NAFLD with ALT elevation [24,25]. In this regard, several researchers have suggested that ALT elevation and/or NAFLD may be a hepatic component of metabolic syndrome [6,7].
In the present study, we used waist-to-height ratio instead of BMI in order to determine the association of ALT with anthropometric measures due to the fact that in the current study, ALT level showed a higher correlation coefficient with waist-to-height ratio (rho= 0.66, P<0.001) than BMI (rho=0.579, P<0.001). In this respect, there are studies suggesting that waist-to-height ratio is more useful in screening for cardiovascular risk factors in children and adolescents [26-29]. This measure is much easier to calculate than BMI, does not require sophisticated tables, and can be utilized to determine visceral obesity, even in normal-weight subjects [30]. In addition, waist-to-height ratio integrates waist circumference as a measure of abdominal obesity and adjusts for a subject’s body size by dividing their height [31]. It incorporates the advantages of both BMI and waist-to-hip ratio by accounting for height and abdominal adiposity [32]. Waist-to-height ratio further indicates cardiometabolic risk based on BMI percentile [33]. Furthermore, our study is unique in that we enrolled 12- to 13-year-old young adolescents as subjects and measured their Tanner stages to take the hormonal effect into consideration. Young adolescence, twelve to fourteen years old, is a pivotal stage for constituting healthy lifestyle habits and the majority of the patterns constructed during this expanding period persist well into adulthood [34]. Accordingly, it is of critical significance that pediatricians recognize young adolescents at risk of obesity and/or metabolic abnormalities such as insulin resistance at an early stage of development and adjust their style of living, such as dietary patterns.
However, the present research has a few inherent limitations. First, the epidemiological, cross-sectional method of this research does not allow for a causal inference. Second, we did not implement ultrasonography on participants with elevated ALT for the diagnosis of NAFLD; however, previous research have reported that the association between ALT or AST and fatty liver disease is so precise that elevated ALT and/or AST can be used as a surrogate marker for suspected fatty liver [3,4,35].
In conclusion, we have demonstrated that higher serum ALT concentrations are found in the presence of overweight/obesity in apparently healthy young adolescents and that serum ALT concentrations are significantly and positively associated with waist-to-height ratio, systolic BP, and HOMA-IR. More intensified clinical screening strategies and interventions are warranted for adolescents at risk, particularly those with abdominal obesity, in order to lower the risk of liver disease at an early stage in young adolescents.
Notes
No potential conflict of interest relevant to this article was reported.