Surfactant preparations for preterm infants with respiratory distress syndrome: past, present, and future
Article information
Abstract
Following the first successful trial of surfactant replacement therapy for preterm infants with respiratory distress syndrome (RDS) by Fujiwara in 1980, several animal-derived natural surfactants and synthetic surfactants have been developed. Synthetic surfactants were designed to overcome limitations of natural surfactants such as cost, immune reactions, and infections elicited by animal proteins contained in natural surfactants. However, first-generation synthetic surfactants that are protein-free have failed to prove their superiority over natural surfactants because they lack surfactant protein (SP). Lucinactant, a second-generation synthetic surfactant containing the SP-B analog, was better or at least as effective as the natural surfactant, suggesting that lucinactant could act an alternative to natural surfactants. Lucinactant was approved by the U. S. Food and Drug Administration in March 2012 as the fifth surfactant to treat neonatal RDS. CHF5633, a second-generation synthetic surfactant containing SP-B and SP-C analogs, was effective and safe in a human multicenter cohort study for preterm infants. Many comparative studies of natural surfactants used worldwide have reported different efficacies for different preparations. However, these differences are believed to due to site variations, not actual differences. The more important thing than the composition of the surfactant in improving outcome is the timing and mode of administration of the surfactant. Novel synthetic surfactants containing synthetic phospholipid incorporated with SP-B and SP-C analogs will potentially represent alternatives to natural surfactants in the future, while improvement of treatment modalities with less-invasive or noninvasive methods of surfactant administration will be the most important task to be resolved.
Introduction
The first successful trial of surfactant replacement therapy in preterm infants with respiratory distress syndrome (RDS) was reported by Fujiwara et al. [1] using surfactant-TA in 1980. Surfactant-TA (Surfacten, Tokyo Tanabe Co, Tokyo, Japan) is a modified minced bovine lung surfactant extract that contains surfactant protein (SP)-B and SP-C with dipalmitoyl phosphatidyl-choline (DPPC), tripalmitin, and palmitic acid. Surfactant-TA can improve neonatal morbidity such as pneumothorax, intracranial hemorrhage, bronchopulmonary dysplasia (BPD), and mortality of preterm infants associated with RDS [2].
Various animal-derived natural surfactants, first-generation synthetic surfactants, and second-generation synthetic surfactants have been developed. However, individual neonatal intensive care units use different surfactants. Various natural surfactants and synthetic surfactants will be compared from the past to present in this review. In addition, better preparations or mode of administration of surfactants will be suggested to improve outcome of preterm infants.
Composition and functions of pulmonary surfactants
The function of pulmonary surfactants is essentially to lower surface tension, thus preventing collapse of alveoli at the end of expiration. The surfactant is composed of a complex mixture of approximately 90% lipids and 10% proteins. These lipids include 80%–90% phospholipids, 5% neutral lipids, and cholesterol. The phospholipids are mainly composed of 80% phosphatidyl-choline, 5%–10% phosphatidyl-glycerol (PG), and other phospholipids. Major surface-active phospholipids that can lower surface tension include DPPC and PG [3,4].
SPs are composed of 2 hydrophobic proteins, SP-B and SP-C, and 2 hydrophilic proteins, SP-A and SP-D. SP-B and SP-C play significant roles in the adsorption and spread of DPPC to stabilize alveoli. Phospholipids incorporated with SP-B and SP-C and packaged with lamellar bodies are secreted into the airspace. Phospholipids layers called surface films are formed at the air-liquid interface and SP-B and SP-C also help stabilize this surface film during respiration. DPPC can adsorb to the air-liquid interface of alveoli through hydrophilic head groups with affinity to water and with the hydrophobic tail toward air, thus reducing surface tension [5].
The surfactant storage pool in term newborn infants is 100 mg/kg whereas that in preterm infants is 4–5 mg/kg at birth. Thus, exogenous surfactant replacement therapy in preterm infants is crucial until endogenous surfactant levels are sufficient to stabilize the alveoli and reduce surface tension [6].
First-generation synthetic surfactant
1. Composition of protein-free first-generation synthetic surfactants
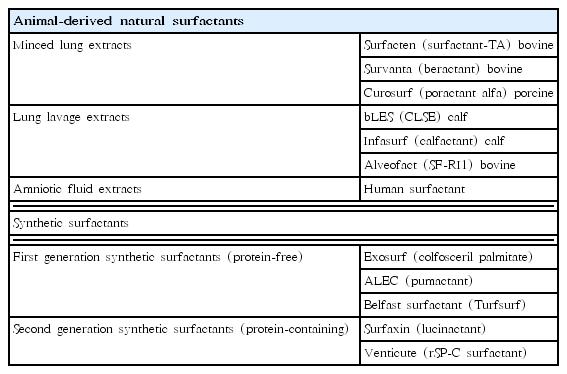
The benefits of exogenous surfactants for preterm infants with RDS are well established. However, animal-derived natural surfactants have limitations such as their elevated costs and limited production due to animal availability. In addition, they contain animal proteins that may be potentially immunogenic and infectious. Therefore, synthetic surfactants have been developed to overcome these limitations of natural surfactants. Synthetic surfactants are manufactured with fewer production limitations. In addition, they do not contain immunogens or pro-inflammatory mediators that cause BPD and animal-borne infections because they are free of animal proteins [7]. First-generation synthetic surfactants contained phospholipids only without SPs. Commonly used protein-free, first-generation synthetic surfactants were colfosceril palmitate (Exosurf, GlaxoSmithKline, Brentford, UK) and pumactant (ALEC, artificial lung expanding compound, Britannia Pharmaceuticals Ltd., Reading, UK) (Table 1).
2. Comparison of first-generation synthetic surfactant with natural surfactant
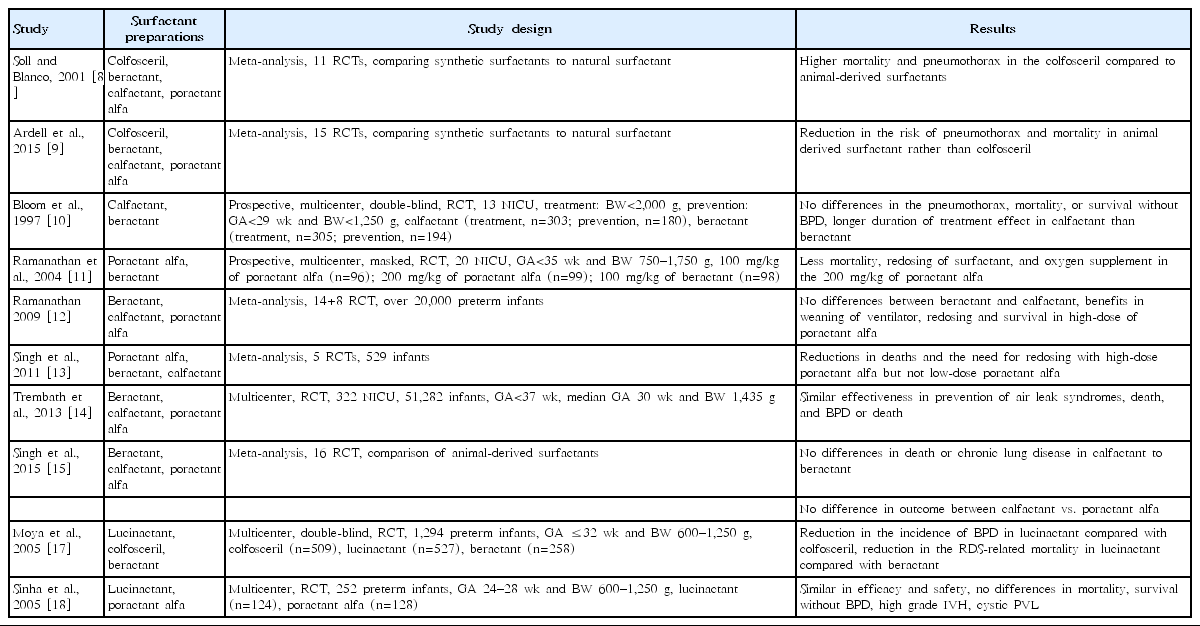
Multiple studies have been conducted to compare natural surfactants with protein-free, first-generation synthetic surfactants (Table 2). Soll and Blanco reviewed 11 randomized controlled trials (RCTs) (from 1975 to 2000) and compared protein-free synthetic surfactants with natural surfactants [8]. Protein-free synthetic surfactants failed to lower surface tension whereas natural surfactants reduced the requirement for ventilator support, the risk of pneumothorax, and the risk of mortality [8]. Protein-free synthetic surfactants have been associated with increased mortality with greater risk of pneumothorax than animal-derived surfactants. The inferiority of protein-free synthetic surfactants might be attributable to the absence of SP-B and SP-C, resulting in failure to lower surface tension.
Ardell et al. [9] reviewed 15 RCTs (from 1975 to 2014) comparing protein-free synthetic surfactants to natural surfactants. Greater early improvement in the requirement of a ventilator, fewer cases of pneumothorax, and fewer deaths have been associated with natural surfactants. This superiority of natural surfactants over protein-free synthetic surfactants was directly related to their SP-B and SP-C content. Finally, protein-free, first-generation synthetic surfactants have been removed from the market, as the superiority over natural surfactants could not be demonstrated.
Natural surfactants
1. Composition of animal-derived natural surfactants
Beractant (Survanta, Abbott Laboratories, Abbott Park, IL, USA) is used in Western regions such as the United States and Europe instead of surfactant-TA. Beractant is similar to surfactant-TA. It is a modified minced bovine lung surfactant extract with SP-B, SP-C, DPPC, tripalmitin, and palmitic acid. Several natural surfactants have become available from various manufacturers following the synthesis of surfactant-TA. Calfactant (Infasurf, ONY Inc., Amherst, NY, USA) was derived from calf lung lavage extract, while poractant alfa (Curosurf, Chiesi Farmaceutici, Parma, Italy) was synthesized from minced porcine lung extract.
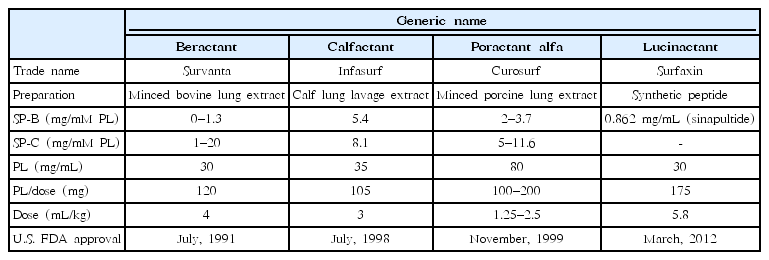
After beractant was approved by the U.S. Food and Drug Administration (FDA) in July 1991, calfactant was approved in July 1998, followed by poractant alfa in November 1999. These agents differ in their preparation, SP concentration, phospholipid concentration, and volume of administration (Table 3). The concentration of SP- B is the highest in calfactant, followed by that in poractant alfa, while the concentration of SP-B is the lowest in beractant [7]. The concentration of phospholipid is the highest in poractant alfa. Volumes of administration per kilogram of body weight for beractant, calfactant, and poractant alfa are 4 mL, 3 mL, and 2.5 mL (initial dose)/1.25 mL (subsequent dose), respectively.
2. Comparison of animal-derived natural surfactants
There have been various comparative studies evaluating natural surfactants (Table 2). Results differ from center to center. In 1997, Bloom et al. [10] reported that there were no significant differences in the incidence of pneumothorax, mortality, or survival without BPD between calfactant and beractant, although calfactant seemed to have a longer duration of treatment effect than beractant. Calfactant was approved by the U.S. FDA the following year. Ramanathan et al. [11] compared the efficacy and safety of poractant alfa and beractant in preterm infants with RDS (3 groups: 100 mg/kg of poractant alfa, 200 mg/kg of poractant alfa, and 100 mg/kg of beractant). Mortality, redosing of surfactant, and oxygen supplements were significantly reduced in the 200 mg/kg of poractant alfa group than in the 100 mg/kg of poractant alfa or beractant groups. Several years later, Ramanathan [12] reviewed 8 trials comparing natural surfactants and concluded that poractant alfa was associated with lower mortality, less redosing of surfactant, and oxygen supplement compared to calfactant or beractant. However, these differences may be related to the higher amount of phospholipids and plasmalogens present in 200 mg/kg of poractant alfa group.
Singh et al. [13] reviewed 5 RCTs to compare the efficacy of a porcine surfactant (poractant alfa) and bovine surfactants (beractant and calfactant). There were significant reductions in mortality and redosing requirement of surfactants in high-dose 200 mg/kg of poractant alfa, but not in low-dose poractant alfa. There were no differences between the porcine surfactant and bovine surfactants.
Trembath et al. [14] reported that pneumothorax, mortality, and BPD were similar for beractant, calfactant, and poractant alfa. They proposed that the previously described differences in outcomes between surfactants might be due to study site variations and not to actual differences in efficacy.
Because of the wide range of differences in the efficacy of natural surfactants, Singh et al. [15] conducted a systematic review of 16 RCTs comparing natural surfactants. Seven treatment trials and 2 prevention trials comparing a bovine lung lavage surfactant (calfactant) to a modified bovine minced lung surfactant (beractant) were reported. There were no differences in death or chronic lung disease in the prevention or treatment trials. There were no differences in outcomes between the bovine lung lavage surfactant (calfactant) and the porcine minced lung surfactant (poractant alfa). There have been 9 treatment trials comparing a modified bovine minced lung surfactant (beractant) to a porcine minced lung surfactant (poractant alfa). Mortality, oxygen requirement, redosing need, and patent ductus arteriosus (PDA) requiring treatment were higher in beractant-treated patients than in poractant alfa-treated patients. However, mortality and oxygen requirement decreased only with high-dose (200 mg/kg) poractant alfa.
Second-generation synthetic surfactant
SP-B and SP-C play a significant role in the adsorption and spread of DPPC from the aqueous phase to form a monolayer along the air-liquid interface and in stabilizing alveoli [16]. Older-generation synthetic surfactants not containing SPs have been removed from the market because they failed to reduce mortality and pneumothorax associated with RDS.
1. Lucinactant: protein-containing second-generation synthetic surfactant
1) Composition of lucinactant
Lucinactant (Surfaxin, Discovery Laboratories, Warrington, PA, USA) is a second-generation synthetic surfactant that contains a synthetic peptide resembling SP-B called sinapultide. Sinapultide is a 21-amino-acid hydrophobic synthetic peptide consisting of leucine (L) and lysine (K) repeating units (KL4). The concentration of sinapultide in lucinactant is higher than the concentration of SP-B in natural surfactants. It has greater resistance to oxidation and protein inhibition. Thus, it can improve pulmonary function of preterm infants with RDS.
2) Comparison of lucinactant to natural surfactant
There have been two multicenter RCTs comparing lucinactant with natural surfactants (Table 2). The Safety and Effectiveness of Lucinactant Versus Exosurf in a Clinical Trial (SELECT) was conducted by the International Surfaxin Collaborative Study Group [17]. They enrolled 1,294 preterm infants assigned randomly to colfosceril palmitate (n=509), lucinactant (n=527), or beractant (n=258). Lucinactant reduced RDS-related mortality compared to both colfosceril palmitate and beractant. Lucinactant reduced BPD at 36 weeks of postmenstrual age compared to colfosceril palmitate. It also reduced the mortality rate compared to beractant.
Another multicenter RCT, Surfaxin Therapy Against RDS (STAR), was conducted by the STAR Collaborative Group [18]. They enrolled 252 preterm infants and assigned them to lucinactant (n=124) or poractant alfa (n=128) group. There were no significant differences in mortality, survival without BPD, intraventricular hemorrhage (IVH) (grades 3 and 4), or cystic periventricular leukomalacia (PVL) between lucinactant- and poractant alfa-treated patients. The authors concluded that lucinactant was as safe and effective as poractant alfa.
Pulmonary and neurodevelopmental outcomes of preterm infants enrolled in the SELECT and STAR trials were followed up through to 1 year of corrected age [19]. The incidence of postdischarge rehospitalization or of respiratory illnesses such as cough, wheezing, and pneumonia did not differ between surfactant groups in the SELECT trial and the STAR trial. Muscle-tone abnormalities were fewer in the lucinactant group than in the colfosceril and beractant groups. Gross motor delay was also less frequent in the lucinactant group than in the colfosceril group. However, the incidence of gross tone, reflex abnormalities, blindness, or deafness at the 1-year corrected age did not differ between the lucinactant and natural surfactant groups.
Lucinactant reduced RDS-related mortality compared with beractant. It also reduced BPD at 36 weeks of postmenstrual age compared with colfosceril palmitate. Lucinactant also reduced mortality rates compared with beractant in another multicenter study [20]. Consecutive studies have demonstrated that lucinactant was better or at least as effective as natural surfactant in efficacy and safety, suggesting that lucinactant could be used as an alternative to natural surfactants [21,22].
3) FDA approval of lucinactant
Lucinactant is the first U.S. FDA-approved protein-containing synthetic surfactant. It was approved by the U.S. FDA in March 2012 as the fifth surfactant to treat neonatal RDS following colfosceril palmitate (Exosurf), beractant (Survanta), calfactant (Infasurf), and poractant alfa (Curosurf). Lucinactant contains 30 mg of phospholipids per mL with a recommended dose of 5.8 mL/kg of body weight (i.e., 175 mg of phospholipids per kg of body weight) (Table 3). It is a gel structure in origin; thus, it has to be warmed at 44°C for 15 minutes followed by vigorous shaking to generate a uniform free-flowing suspension.
2. Other second-generation synthetic surfactants: CHF5633
CHF5633 is the first synthetic surfactant containing analogs of both SP-B and SP-C. Sato and Ikegami treated preterm lambs with CHF5633, survanta, or air [23]. All lambs treated with air died of respiratory difficulty. The CHF5633 group had a faster initial response of tidal volume than the survanta group. The CHF5633 group had a higher compliance than the survanta group at 20 minutes and 300 minutes of age, with a higher lung volume at 10 cmH2O, than the survanta group. Alveoli were uniformly expanded in the CHF5633 group, somewhat atelectatic in the survanta group, and atelectatic in the air group based on hematoxylin and eosin staining. Inflammatory mediators such as interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor alpha mRNA expression levels were not significantly different between the CHF5633 and survanta groups. The authors concluded that CHF5633 was effective in treating preterm lambs with surfactant deficiency [23].
Sweet et al. [24] conducted a first-in-human multicenter cohort study with CHF5633 in 40 preterm infants (27+0 to 33+6 weeks of gestation) diagnosed with RDS from 12 European centers in the United Kingdom, Czech Republic, and Germany. Both mean airway pressure and FiO2 improved rapidly and were sustained. There was no systemic absorption or immunogenicity supported by undetectable peptides or antibodies. There were no significant adverse events associated with CHF5633, except for one episode of endotracheal tube obstruction with 200 mg/kg of CHF5633. The authors concluded that both 100 and 200 mg/kg of CHF5633 were effective and safe for preterm infants with RDS. However, larger RCTs with more preterm infants are needed to support this result.
Surfactants used in Korea
In Korea, surfactant-TA was introduced to the market in 1990 while poractant alfa entered the market in 2002 and calfactant in 2009. One published study enrolled 332 preterm infants at 24–31 weeks of gestation with RDS and compared the efficacy of calfactant with surfactant-TA and poractant alfa, the most commonly used surfactants in Korea [25]. Surfactant redosing, pulmonary air leaks, duration of mechanical ventilation, PDA, IVH (grades 3 and 4), PVL, or mortality was not different among groups. However, pulmonary hemorrhage and moderate to severe BPD were slightly increased in patients treated with poractant alfa. Thus, calfactant, surfactant-TA, and poractant alfa were equally effective in this study. However, further randomized prospective studies comparing these surfactants are needed.
Surfactant was administered as rescue therapy for preterm infants diagnosed with RDS until December 2010. As of January 2011, surfactant has been administered as prophylactic therapy in infants born at <30 weeks’ gestation or with a birth body weight ≤1,250 g within 2 hours after birth, according to notification No. 2010-135 from the Ministry of Health and Welfare on January 2011.
Other considerations regarding use of surfactant preparations
There are several natural or synthetic surfactants with different concentrations of SP and phospholipids as well as dosing indications. The reported efficacies differ based on the preparations. However, these differences might be related to variations in study sites rather than actual differences in efficacy [26].
It has been reported that the timing of administration of the surfactant, such as prophylactic versus rescue and early (within 2 hours after birth) versus delayed (later than 2 hours after birth) treatment, is more important than the composition of the surfactant preparations themselves [27]. Moreover, the mode of surfactant administration is also more important than the composition of the surfactant preparations. A noninvasive ventilator may improve the pulmonary outcome of preterm infants [28]. Less invasive surfactant administration was attempted by the continuous positive airway pressure or intubation at birth trial [29] and the SUPPORT (Surfactant, Positive Pressure, and Pulse Oximetry Randomized Trial) study [30]. Less invasive surfactant administration with intubation and surfactant administration followed by immediate extubation to nasal respiratory support (InSurE) reduced the need of ventilator support [31]. New modes of surfactant administration such as Minimally-Invasive Surfactant Therapy, Non-Invasive Surfactant Therapy, and aerosolized delivery of surfactants have been developed to reduce the risks associated with endotracheal tube placement [27].
Conclusion
Outcomes of surfactant administration in preterm infants with RDS depend on various conditions of preterm infants. A new synthetic surfactant containing a synthetic phospholipid incorporated with synthetic peptides resembling SP-B and SP-C may represent a potential alternative to animal-derived natural surfactants to treat preterm infants with RDS in the coming years. Improvement in treatment modality by applying a less invasive or a noninvasive method of surfactant administration will be the most important task to be resolved in the near future.
Notes
No potential conflict of interest relevant to this article was reported.