Bronchopulmonary dysplasia: how can we improve its outcomes?
Article information
Abstract
Bronchopulmonary dysplasia (BPD) is a chronic lung disease of preterm infants with multiple factors affected from prenatal to postnatal periods. Despite significant advances in neonatal care over almost 50 years, BPD rates have not decreased; in fact, they may have even increased. Since more preterm infants, even at periviable gestational age, survive today, different stages of lung development affect the pathogenesis of BPD. Hence, the definition of BPD has changed from “old” to “new.” In this review, we discuss the various definitions of BPD, risk factors from the prenatal to postnatal periods, management strategies by phase, and future directions for research.
Key message
The important messages of this article are bronchopulmonary dysplasia is still a problem in the neonatology filed and hence various efforts are needed to decrease the incidence and improve its outcome.
Introduction
Bronchopulmonary dysplasia (BPD) is a chronic multifactorial respiratory disease related to lung injury in preterm infants [1]. The very first description of BPD was provided by Northway et al. [2], and the clinical and radiological features of premature infants who had developed respiratory distress syndrome (RDS) with prolonged high inspired concentrations of oxygen ventilation were originally described [3].
The incidence of BPD remains unchanged or is increasing owing to an increasing survival rate of extremely preterm infants, although it has definitely decreased in preterm infants delivered at >28 weeks’ gestation [4-6]. In Korea, the incidence was 28.9% in 2015 [7].
With the tremendous development of various treatment modalities including antenatal steroids, early surfactant administration, strict fluid control, and less aggressive ventilation techniques, the so-called old (or classic) definition of BPD is rarely seen nowadays. The pathophysiology of BPD in very low birthweight (VLBW) infants is thought to be a series of processes between lung injury and repair during lung development over weeks to months; as such, the concept of “new” BPD has come to the forefront [3,8]. Perhaps inevitably, various attempts have been made to define diagnostic criteria over almost 50 years [9-12]. The definition of BPD revised by the National Institute of Child Health and Human Development (NICHD) in 2000 stated that the definition should distinguish between infants with a gestation of ≤32 weeks versus >32 weeks of postmenstrual age (PMA) and rate disease severity [13].
BPD causes long-term complications in terms of respiratory, cardiovascular, and neurological development from early childhood to adulthood and is a major public health problem in the end [14]. Despite a wide variety of studies on its pathophysiology, prevention methods, and management modalities, BPD treatment options remains limited.
This review summarizes the different definitions of BPD used in recent studies of its pathophysiology and its current treatment status and discusses recent newer therapies to improve outcomes of VLBW infants.
Changes in BPD definition
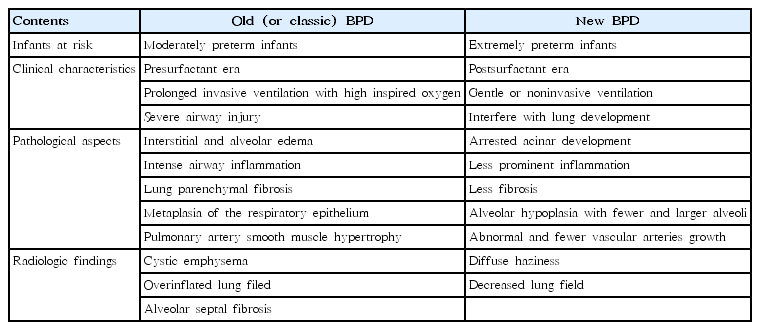
In its first description in the 1960s, BPD was reported in a group of moderately preterm infants with surfactant deficiency who survived with adverse outcomes from prolonged ventilation with a high oxygen supply. Hence, severe airway and parenchymal injury with alveolar cell hyperplasia, bronchiolar squamous metaplasia causing emphysema, and septal fibrosis were the typical radiologic and pathologic features of classic BPD [8,14].
Lung radiologic changes were also considered for the definition of BPD together with supplemental oxygen support in the late 1970s [8,11]. In one study, high-resolution computed tomography showed linear and triangular opacities, air trapping, and mosaic perfusion [15]. Another study reported that preterm infants born at approximately 26 weeks in transient changes in lung development from canalicular to saccular stage had greater lung parenchymal changes [16].
With exogenous surfactant therapy and antenatal corticosteroids, preterm infants who might have died previously can now survive well even after neonatal intensive care unit (NICU) discharge. In the late 1980s, Shennan et al. [10] suggested a simple definition of BPD for VLBW infants as the use of oxygen supplementation at 36 weeks of PMA; this concept predicted a poor outcome at 2 years of age.
Owing to the advances in the field of neonatology, the survival of extremely preterm infants who are born at the late canalicular-saccular stage of lung development has increased. Early surfactant administration with less invasive ventilation techniques in these groups decreased the oxygen requirement at the time of assessment; hence, it was difficult to categorize them as having classic BPD [3,17].
The definition by NICHD [13], called “new” BPD, separates infants with aged ≤32 from those >32 weeks PMA and proposed a severity-based definition. Severity was categorized for preterm infants in need of supplemental oxygen for ≥28 days as: mild BPD, room air at 36 weeks PMA or at discharge; moderate, supplemental oxygen <30% at 36 weeks PMA or at discharge; and severe, supplemental oxygen ≥30% and/or need for positive pressure at 36 weeks PMA or at discharge. A very recent subclassification of severe BPD divides it into 2 phenotypes: Type 1 is relatively less severe and includes infants using a high-flow nasal cannula or continuous positive airway pressure at 36 weeks PMA, while type 2 is relatively more severe and includes those on mechanical ventilation [18]. The differences between “old” and “new” BPD are summarized in Table 1.
From another point of view, BPD is divided into early, evolving, and established BPD with a step-by-step therapeutic approach: early, very early time from birth up to the 1st week of life; evolving, weeks 1–36 PMA; and established, >36 weeks PMA [19].
The reason why a uniform definition of BPD is lacking is that it is based on a clinical aspect of treatment with oxygen only and does not consider radiology or laboratory test or histopathologic findings. This could limit the comparisons between studies on BPD.
Risk factors
A known multifactorial disease, BPD first occurs at the start of lung development in the prenatal period to the postnatal period after birth and persists even after NICU discharge. In addition, VLBW infants do not have “normal” lungs, so they are very vulnerable to injury.
Inflammatory responses as an accumulation of neutrophils and macrophages in the injured lung together with growth factor elaboration and cytokines increased vascular permeability which resulted in the development of BPD [20]. Numerous inflammatory biomarkers in the amniotic fluid as well as tracheal aspirates of neonates have been studied to identify early detectable markers for infants who would subsequently develop BPD [19].
Chorioamnionitis, a common known factor for preterm delivery, is an important major antenatal factor contributing the development of BPD [21]. Although clinical chorioamnionitis sometimes does not correlate with histological chorioamnionitis or culture-proven amniotic fluid infection, it represents a maternal and/or fetal inflammatory response [22]. Although causes of chorioamnionitis vary, infants delivered before 30 weeks’ gestation showed amniotic fluid with Ureaplasma and Mycoplasma infection and the most common isolated microorganism is Ureaplasma [21,23]. Ureaplasma colonization or infection plays an important role in the pathogenesis of BPD, reducing the colonization burden with prophylactic antibiotics or postnatal therapeutic treatment might be considered [24]. Recent studies showed that genetic and/or environmental factors are contributors to the development of BPD [25,26]. A familiar tendency and heritability in twin studies demonstrated genetic susceptibility associated with BPD, while many other studies focused on identifying specific gene mutations associated with lung development, immunity, and oxidative stress with BPD [27].
The initial resuscitation technique just after delivery is an important factor as well since the preterm lung can be easily damaged by mechanical ventilation. Some studies showed that if initial resuscitation for preterm infants was started with a large tidal volume, lung injury could occur, and increased pro-inflammatory cytokine levels were seen with mechanical ventilation in surfactant-treated preterm lungs [28,29]. To avoid such a secondary ventilator-associated lung injury, variable ventilation support with early surfactant treatment is undoubtedly needed. Various factors for initiation of ventilation such as tidal volume, end-expiratory pressure, oxygen concentration, humidity, and temperature also affect lung injury [22,29].
Other known factors affecting the development of BPD are intrauterine growth retardation, multiple births, patent ductus arteriosus, nosocomial infection or postnatal sepsis, and surgical necrotizing enterocolitis [29]. These various factors sometimes independently or cooperatively affect different phases and may affect BPD severity in individual infants.
Prevention and treatments
Preventing and treating the development of BPD requires a multidisciplinary approach from the prenatal period—involving an obstetrician—to the postnatal period—involving several other experts including a respiratory therapist, cardiologist, gastroenterologist, nutritionist, rehabilitation physician, expert neonatal nurses, and even social workers in addition to neonatologists.
1. Antenatal care
The best way to prevent the development of BPD is to prevent preterm delivery. Pharmacological interventions such as the administration of antibiotics, use of vaginal prostaglandin suppository, tocolytic agents such as magnesium sulfate, and surgical interventions like cervical cerclage for cervical incompetence may also prevent preterm birth [30,31].
The administration of antenatal corticosteroid before delivery promotes the maturation of surfactant, thereby reducing the incidence of RDS; however, whether it decreases the incidence of BPD is still unclear. A recent systematic review concluded that use of antenatal steroids effectively accelerated fetal lung maturation in women at risk of preterm birth regardless corticosteroid type or amount [32].
In the delivery room in which a premature birth is imminent, a team approach with on-site tertiary neonatal facilities is definitely needed to improve mortality and long-term morbidity rates [3,33]. The initial stabilization of infants at risk for RDS with continuous positive airway pressure (CPAP), sustaining lung inflation, and prudent titration of supplemental oxygen during resuscitation to achieve targeted oxygen saturation are recommend with close observation of heart rate, chest wall movement, and preductal saturations [33,34].
2. Postnatal care
After delivery, the therapeutic approach should focus on 2 aspects: what ventilation technique should be applied and what pharmacologic treatment should be administrated in each of the 3 phases.
Since preterm lungs are highly vulnerable to barotrauma and volutrauma, less invasive ventilation like avoiding endotracheal intubation is known as a promising treatment modality for reducing the development of BPD [35]. Even when transferring preterm infants who require positive pressure ventilation from delivery room to NICU, the use of CPAP therapy with a T-piece resuscitator (Neopuff; Fisher and Paykel Healthcare, Auckland, New Zealand) rather than a self-inflating bag is recommended to reduce the risk of BPD development [36].
As mentioned above, avoiding unnecessary intubation is the best way to decrease the incidence of BPD from the respiratory care point of view. However, to prevent RDS, most preterm infants need exogenous surfactant administration on a prophylactic or early rescue basis. Hence, alternate noninvasive or minimally invasive routes have been introduced [17,37]. For instance, aerosolized surfactant delivered by a nebulizer was attempted despite proof of its lack of efficacy. Other methods were surfactant administration via a laryngeal mask airway or administration directly to the trachea via a thin catheter such as the less invasive surfactant administration technique or minimally invasive surfactant therapy. Intubation and surfactant administration followed by immediate extubation could be an alternative modified strategy with alveolar recruitment maneuver before surfactant administration could be another new method.
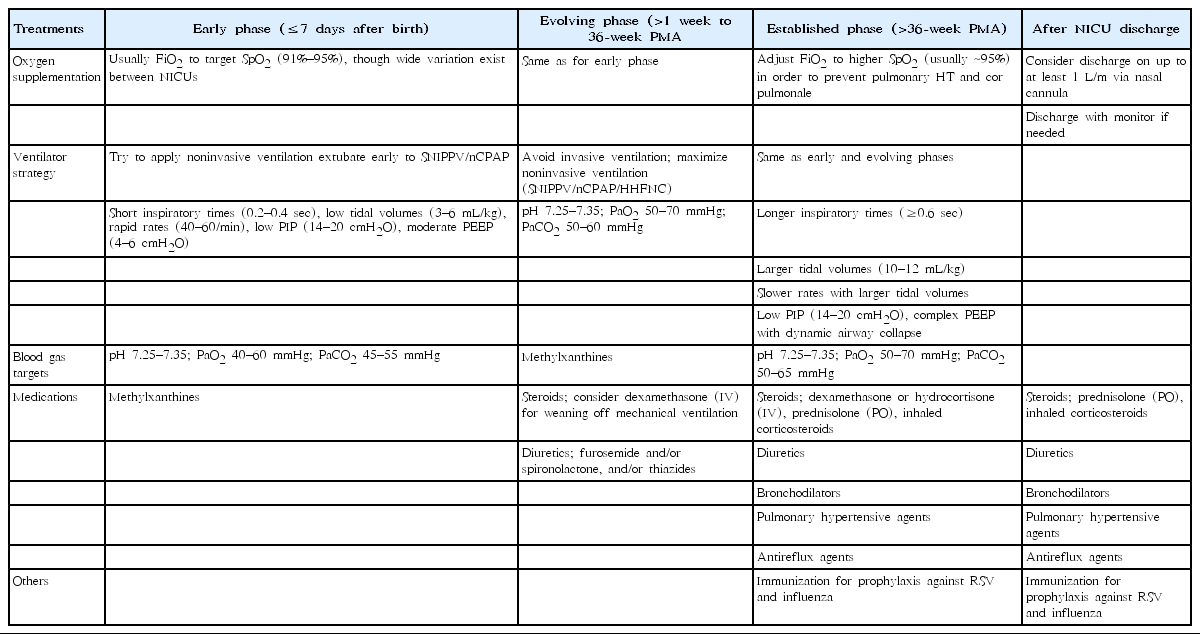
In the early period before BPD is established, oxygen should be maintained at 91%–95% to avoid oxygen toxicity. However, once BPD is established, oxygen supplementation is widely accepted with variation (even >95%) across centers to prevent pulmonary hypertension and cor pulmonale [38].
Appropriate ventilation strategies are perhaps most important for controlling the development of BPD. In the early phase, especially in the first week of life, one must try extubation and apply nasal CPAP or synchronized nasal intermittent positive pressure ventilation to minimize acute lung injury while maintaining a low tidal volume (3–5 mL/kg), short inspiratory times (0.2–0.4 seconds), low peak inspiratory pressure (14–20 cmH2O), and moderate positive end-expiratory pressure (4–6 cmH2O) [18,19]. At the evolving phase, i.e., at 1–36 weeks’ PMA, the aim of respiratory care should be optimizing adequate gas exchanges, reducing the work of breathing, and healing the injured airway [18]. Avoiding intubation and maximizing noninvasive ventilation using CPAP, SIPPV, and high-flow nasal cannula remain effective strategies for decreasing lung injury [18,19]. To achieve this goal, volume-targeted ventilation has more advantages over pressure-supported ventilation, and a recent study recommended adjusting the tidal volume target to 7 mL/kg to reduce the work of breathing [39].
When BPD is established, permissive hypercapnia is allowed to facilitate weaning and optimize adequate gas exchange, reducing the work of breathing and healing the injured lungs as similar to that in the evolving phase. The special features of this phase are different combinations of lung regions with different airway resistance, compliance, and time constants [18]. Hence, at this point, a larger tidal volume (10–12 mL/kg), longer inspiratory time (≥0.6 seconds), and resolved airways obstruction are needed to promote gas exchange [18,19].
Whether to use or not to use postnatal steroids as well as the time, duration, and type (dexamethasone or hydrocortisone) of steroids that can be used is yet to be determined because the results of long-term adverse effects on neurodevelopmental outcomes vary [40,41]. Recent Cochrane reviews concluded that the use of early (<8 days) systemic corticosteroids and late (≥7 days) inhalation reduced BPD outcomes [42,43]. While routine clinical use of dexamethasone in the first week of life is not recommended, when weaning a patient off mechanical ventilation, low-dose dexamethasone (0.89 mg/kg over 10 days) is effective, as first introduced in the DART study [44]. Another multicenter randomized double-blind placebo-controlled study, the PREMILOC, i.e., early prevention of bronchopulmonary dysplasia and neonatal mortality in very preterm infants using low dose of hydrocortisone, proposed the positive effect of increasing the survival rate of extremely preterm infants without BPD using early low-dose prophylactic hydrocortisone [45]. However, the very recent STOP-BPD study (systemic hydrocortisone to prevent bronchopulmonary dysplasia in preterm infants) study performed by the Europe investigating group reported that early low-dose hydrocortisone (cumulative dose, 72.5 mg/kg) initiated between 7 and 14 days after birth showed no favorable outcomes of BPD at 36 weeks’ PMA; hence it did not recommend its use [41]. When using a systemic corticosteroid, one must consider its benefit versus potential short-term adverse effects such as hyperglycemia, systemic hypertension, nosocomial infection, and possible intestinal perforation.
With the idea of lower toxicity, the use of inhaled in addition to systemic corticosteroids has been actively studied [46-49]. A study of early inhaled budesonide with a total of 863 preterm infants <28 weeks PMA administered within 24 hours after birth showed a lower incidence of BPD but a higher mortality rate than the placebo group [46]. A meta-analysis of inhaled corticosteroids including beclomethasone, budesonide, fluticasone, flunisolide, and dexamethasone concluded the possible potential effect, of budesonide in particular, of reducing the risk for BPD but not with mortality and morbidities [47].
Another trial of the combination of an intratracheal corticosteroid–surfactant mixture by Yeh et al. [48] demonstrated a significantly lower incidence of BPD than the surfactant-only–treated group, while a meta-analysis reported a decreased risk of BPD with the administration of an intratracheal budesonide–surfactant mixture [49].
Nutritional support is also a very important part of BPD prevention since preterm infants with BPD have a 15%–25% higher energy requirement than infants without BPD; thus, a high-calorie intake of 140–150 kcal/kg/day is needed to overcome lung injury from oxygen free radicals and promote lung and linear growth [50]. This could be achieved with breast milk or calorically dense formula feeding [18]. Other medications such as diuretics, caffeine, vitamin A, and macrolide antibiotics for a Ureaplasma-colonized respiratory tract as well as nutrition are known to help decrease the incidence of BPD [51] (Table 2). New pharmacological studies are underway on the use of recombinant Clara cell 10-kDa protein, recombinant superoxide dismutase, leukotriene receptor antagonist, inositol, and tocopherol [52,53].
Conclusion
A systematic review of 31 international guidelines for perinatal care demonstrated that near 70% of institutions recommend providing comfort care at 22 weeks’ PMA and active resuscitation at 25 weeks’ PMA [54]. The survival rate of more extremely preterm infants is increasing, especially among those born at 23 or 24 weeks, while survival rates without major morbidity (except BPD) are increasing for infants born at 25–28 weeks, although a difference in survival in seen in periviable live births among countries [6,55]. The great improvements in BPD discussed above have been achieved over almost 50 years; however, additional are needed regarding definitions, deliver room interventions, NICU protocol for evolving BPD, and follow-up management [56]. In addition to general care and practices, special attention is needed for infants with severe BPD.
Notes
No potential conflict of interest relevant to this article was reported.